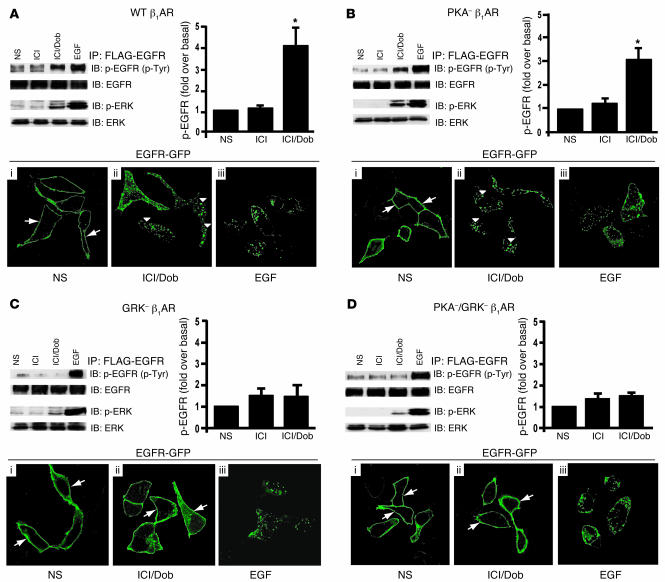
Figure 1. β1AR-mediated transactivation of EGFR requires GRK phosphorylation sites.
HEK293 cells stably expressing WT β1AR (A), PKA– β1AR (B), GRK– β1AR (C), or PKA– GRK– β1AR (D) and transfected with FLAG-EGFR were treated with ICI and Dob (as described in Methods) and compared with cells with no stimulation (NS) or EGF stimulation. WT β1AR (A) and PKA– β1AR (B) induced increases in phospho-EGFR and phospho-ERK1/2 after 5 minutes in response to treatment with Dob, while GRK– β1AR (C) and PKA–/GRK– β1AR (D) lacked this effect; *P < 0.05 versus NS. EGFR internalization following Dob or EGF stimulation for 30 minutes was visualized using confocal microscopic analysis of HEK293 cells stably expressing the above β1AR mutants and transfected with EGFR-GFP. In the absence of agonist, EGFR-GFP was visualized on the membrane in each stable cell line (A–D, i, arrows), while EGF stimulation resulted in redistribution of EGFR-GFP into cellular aggregates (A–D, iii). Treatment of either WT β1AR or PKA– β1AR cells with Dob resulted in a similar redistribution of EGFR into cellular aggregates (A and B, ii, arrowheads), an effect that was absent in GRK– β1AR and PKA–/GRK– β1AR cells, where EGFR-GFP remained on the membrane (C and D, ii, arrows). Original magnification, ×100.