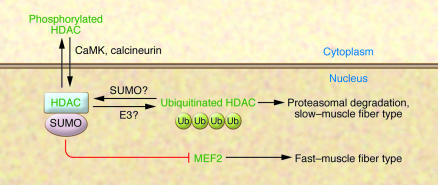
Figure 1. Ubiquitination of class II HDACs causes their degradation via the proteasome, allowing MEF2 to induce a slow–muscle fiber phenotype.
One potential mechanism for regulation of HDAC sensitivity to ubiquitination is illustrated — SUMOylation. One model for MEF2 inactivation is that SUMOylated HDAC, which is associated with the SUMO E2, Ubc9, can associate with MEF2, leading to MEF2 SUMOylation and inactivation. In this issue of the JCI, Potthoff et al. (2) demonstrate that HDACs are degraded via ubiquitination in slow muscle fibers, resulting in active MEF2, which is necessary and sufficient to induce the expression of genes required for the slow–muscle fiber–type transcriptional program. Phosphorylation of the HDACs by CaMK (or dephosphorylation by calcineurin) does not seem, by itself, to perturb the sensitivity of HDAC to ubiquitination, but these proteins regulate HDAC localization to the nucleus; ubiquitinated HDAC proteins are found primarily in the nucleus, perhaps implicating a nuclear-localized E3 ubiquitin ligase. Ub, ubiquitin.