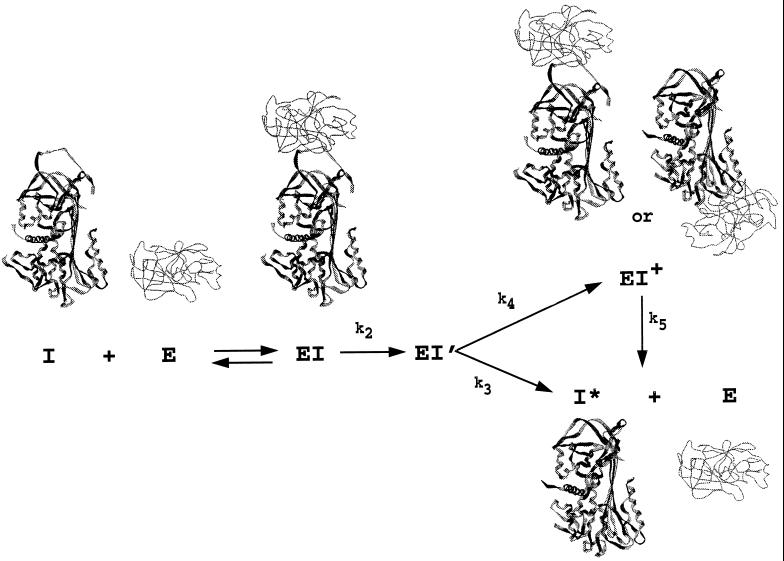
Figure 1.
The branched suicide substrate pathway of serpins (I) interacting with proteinase (E), showing the possible types of structure for the different intermediates and products. The initial noncovalent Michaelis-like complex (EI) is expected to resemble non-serpin proteinase–inhibitor complexes such as those of bovine pancreatic trypsin inhibitor (BPTI) and trypsin. The structure of cleaved serpin (I*; the product of the substrate branch of the pathway) is known for several serpins and has the cleaved reactive center loop completely inserted into β-sheet A. Two different types of structure are shown for the stable complex (EI+). In one, little or no movement of the proteinase has occurred and stabilization results largely from noncovalent interaction with the serpin. In the other, the P1–P1′ peptide bond has been cleaved, permitting complete loop insertion, with the intermediate trapped at the stage of the covalent acyl enzyme intermediate. The EI+ complex can decay very slowly (k5) to cleaved serpin and free proteinase.