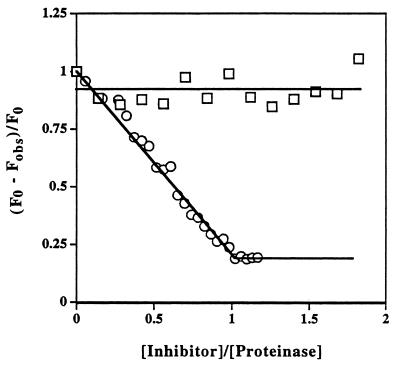
Figure 2.
Ability of inactivated (anhydro-) trypsin to form tight complex with α1-proteinase inhibitor Pittsburgh but not with wild-type α1-proteinase inhibitor. Titration of α1-proteinase inhibitor Pittsburgh (○) into a solution of anhydrotrypsin (4.5 μM) in the presence of p-aminobenzamidine (100 μM) resulted in stoichiometric displacement of the noncovalently bound fluorescent probe, indicating tight 1:1 noncovalent complex formation. The solid straight lines are for visual aid only. Similar titration of wild-type α1-proteinase inhibitor (□) under the same conditions led to negligible complex formation and consequently little change in probe fluorescence.