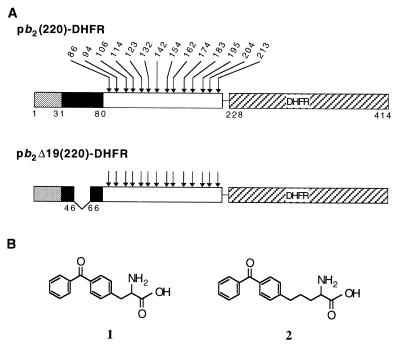
Figure 1.
(A) The fusion protein pb2(220)-DHFR contains the first 220 residues of the cytochrome b2 precursor fused with a 7-residue linker fragment to mouse dihydrofolate reductase (DHFR). The fusion protein pb2 Δ19(220)-DHFR contains the first 220 residues of the cytochrome b2 precursor, lacking residues 47–65 of the presequence, fused with a 7-residue linker fragment to DHFR. The first 31-residue segment of the presequence is cleaved by matrix-localized processing peptidase (MPP) in both proteins, and the second 49-residue segment is cleaved by inner membrane protease I (Imp1p) in pb2(220)-DHFR. The positions of amino acids that were replaced with 1 or 2 are indicated by arrows. The numbering of amino acid residues for pb2(220)-DHFR was used for pb2 Δ19(220)-DHFR so that the crosslinking results (Table 1) can be directly compared. (B) Chemical structures of the photoreactive amino acids dl-2-amino-3-(p-benzoylphenyl)propanoic acid (1) and dl-2-amino-5-(p-benzoylphenyl)pentanoic acid (2).