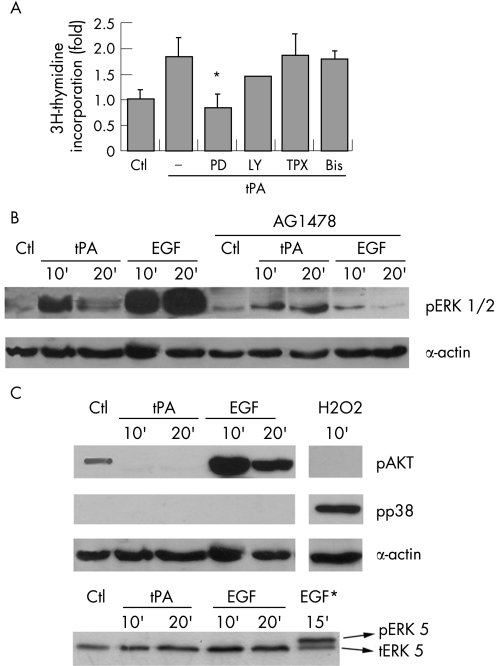
Figure 3 Active ERK1/2 are required for tPA effects on proliferation. (A) Induction of proliferation was analysed by the incorporation of [3H]‐thymidine and expressed as fold over unstimulated serum starved controls (Ctl). Serum starved PANC1 cells were treated with tPA (6 nM) in the presence of PD098059 (PD) 50 μM, LY294002 (LY) 50 μM, pertussis toxin (TPX) 100 μg/ml, and bisindolylmaleimide (Bis) 5 μM. Each treatment is the mean of quadruplicate measurements. At least three independent experiments were performed. The error bars represent the SEM. (*p<0.05). (B) tPA induces phosphorylation of ERK 1/2. Cells were serum starved and treated with tPA (6 nM) or EGF (3 nM) for the indicated times, without or with AG1478. Cell lysates (50 μg) were analysed for ERKs phosphorylation by western blotting with a phospho‐ERK specific antibody. Equal protein loading was verified by re‐analysing the membranes for actin expression levels. A representative blot of three performed is shown. (C) The ability of tPA to phosphorylate AKT (pAKT), p38 MAP kinase (pp38), or ERK5 (pERK5) was analysed by western blotting using specific antibodies to the phosphorylated proteins. The following treatments were performed as controls: H2O2 (1 mM), as a control for p38 MAP kinase phosphorylation; EGF (3 nM), as control for AKT phosphorylation; and HeLa cells treated with 3 nM EGF (EGF*), as a control for ERK5 activation.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.