Abstract
Objective
To evaluate differences between adults who consent to participate in observational research and those who do not.
Design
Prospective, population‐based cohort study.
Setting
35 randomised Irish general practices.
Participants
1609 adults with ischaemic heart disease identified in 2000–1.
Intervention
Medical records search, postal questionnaire and consent form in 2005–6.
Main outcome measures
Differences in demographic and prognostic risk factors between consenters and non‐consenters.
Results
At follow‐up, charts were located for 1592 patients (98.9%). Questionnaires were sent to 1269 patients and 876 were returned (69%). Of these, 574 (65.5%) gave consent for participation in further research. Logistic regression identified four characteristics as independently positively predictive of consent to participation in further research among questionnaire responders: having undergone percutaneous transluminal coronary angioplasty was associated with an increased odds of consent, with an odds ratio (OR) of 1.77 (95% CI 1.09 to 2.86), as was a last recorded blood pressure <140/90 mm Hg (OR = 1.45 (1.00 to 2.09)), a last recorded total cholesterol level <5 mmol/l (OR = 1.71 (1.16 to 2.54)) and being an ex‐smoker rather than a current smoker or non‐smoker (OR = 1.73 (1.17 to 2.57)).
Conclusions
This research demonstrates the potential impact of consent bias in observational research on ischaemic heart disease, a disease of everyday clinical importance in Europe. It demonstrates that clinically important prognostic variables may be associated with consent preferences. Future cohorts, dependent upon prior written consent, may contain disproportionate numbers of those who have made healthy lifestyle decisions, have previously benefited from treatment or whose clinical risk factors are already well managed. As a result, the generalisability of such research may be diminished and the effects of treatments over‐ or underestimated.
Keywords: selection bias, consent, observational research, ischaemic heart disease
In recent years patients' rights to privacy and confidentiality have been increasingly acknowledged both in law and by research ethics committees. Patients' prior written consent is required almost universally where identifiable data are sought for research purposes.1,2,3,4,5,6
The implications for research of the requirement for prior written consent have been widely discussed, but the debate “has largely been confined to professional circles”.7,8,9,10 Research evidence considering public attitudes towards the use of personal medical information in research is limited.1,7,9 Although the Medical Research Council reported that the use of personal data in publicly funded health research is viewed positively by the public “if it will advance medical practice”, and research in Leicestershire reported patients' “altruistic views about participation in research”,1,11 a number of other studies have found that the public would prefer their consent to be sought before access to personal medical data is granted.9,12,13 Research also suggests that there may be only limited understanding among the public of what information is contained in medical records and of the value of such data to research.1,11,13 The promotion of greater understanding appears worthwhile: a study on attitudes to the retention and research use of tissue samples has demonstrated a striking increase in the proportion of patients willing to consent to the use of their personal data in research when the potential value of their doing so was explained.14
The threat posed to observational research by current requirements for prior written consent is grave. Concerns have been expressed among researchers that there is a danger that constraints may have become too strict: that in order to prevent limited and largely theoretical harm to people, work may be prevented which offers large benefits to society.7,13,15,16,17,18,19,20
Among the most serious consequences of the requirement for prior written consent is the threat to the validity of observational research posed by “consent bias”, a term coined to describe the selection bias resulting from the loss of non‐consenters to any cohort. It has been suggested that “patients, the public and professional organisations must consider the implications … before epidemiology and health services research are regarded as too biased to rely on.”15
However, demonstrating the potential seriousness of this phenomenon has only been possible in a small number of studies with access to data for non‐consenters as well as consenters. In Rochester, Minnesota, Jacobsen et al found in their medical records research—to which 79% consented—that those refusing consent were more likely to be female and younger than 60 years. Those with “sensitive” diagnoses such as reproductive disorders, mental disorders or infectious diseases were also less likely to consent to participation.18 A study based on the Registry of the Canadian Stroke Network supported the American finding that women were more likely to refuse consent than men but found that those who did not consent to interview and medical records review (49%) were more likely to be older. In the UK, Angus et al found that different proportions of men and women consented to research participation in different age groups and that consenters were less likely to live in deprived areas.21 Al‐Shahi et al demonstrated that clinically important prognostic variables can be vulnerable to consent bias: among a cohort of people with intracranial vascular malformation, the positive association between one prognostic variable—which often influences the decision to treat in clinical practice—and an important outcome was confirmed when data relating to their whole cohort were analysed, but not so when those who did not give consent (41%) were excluded.15
Methods
The CoHeart study
The CoHeart study is a 5‐year follow‐up of a representative cohort of 1609 people with established ischaemic heart disease (IHD) in 35 randomised and stratified general practices in the west and northwest of Ireland.22 The cohort was established in a cross‐sectional study in 2000–1 and presented a valuable opportunity to conduct a follow‐up study of the secondary prevention of IHD among a representative community cohort at a time when the future of such observational work is at risk. IHD was defined as a history of previous acute myocardial infarction (AMI), angina pectoris, cardiac artery bypass grafting (CABG) or percutaneous transluminal coronary angioplasty (PTCA). With original data available for cohort members irrespective of their consent preferences for research participation, the study also presented an opportunity to test hypotheses relating to consent bias among a large community‐based cohort of people with established IHD.
Ethical approval for the study
Ethical approval for both baseline and follow‐up studies was granted by the Irish College of General Practitioners. Ethical approval at baseline allowed researchers to identify the practice populations of people with IHD through review of general practice records, to establish practice IHD registers, to collect medical and demographic data and to send each member of the cohort a patient questionnaire. The baseline database was subsequently anonymised. Follow‐up data collection was dependent upon the consent and participation of the individual general practitioners (GPs), who are the legal data controllers of patient records in Ireland. Ethical approval allowed for collection of anonymous data from patient records, linked in the practices to baseline data by unique patient identification codes. A protocol was established which enabled the sending of patient questionnaires by agents of the practices without the need to disclose patients' identities to follow‐up researchers. To facilitate future follow‐up, a consent form was enclosed with the patient questionnaire which requested patients' written consent and identification for involvement in further research.
Analysis of consent bias
Patient questionnaires could be returned with or without the consent form—or not returned at all. Thus the study identified three subgroups of patients with different consent preferences: “consenting responders” (who completed the questionnaire and gave their consent and contact details for participation in further research), “non‐consenting responders” (who completed the questionnaire but did not consent) and “non‐responders”.
These subgroups represented different preferences relating to participation in research and consent for access to personal medical data and thus afforded an opportunity to consider associations between these preferences and demographic, medical and healthcare data.
Statistical methods
The selection of variables considered in this study was informed by sources of consent bias identified in the literature: age (by 10‐year age bands), sex, socioeconomic status (measured by eligibility or ineligibility for free General Medical Services (GMS) within the Irish health system—at the time of the follow‐up study just under 40% of the population in the area in which the study was based were GMS eligible, representing the least affluent members of society), and prognostic risk factors. Prognostic risk factors for subsequent IHD events which were considered in analysis were previous AMI, previous CABG or previous PTCA (whether or not patients had ever experienced these events), blood pressure and cholesterol management (last reading at follow‐up less than or equal to or greater than the level recommended by the Second Joint Task Force of the European and other Societies on Coronary Prevention23,24), body mass index (BMI; ⩽25 kg/m2 or >25 kg/m2), self‐reported smoking status (current, ex‐ or non‐smoker at follow‐up) and exercise behaviour (less than or equal to or greater than the level recommended by the Second Joint Task Force, measured by the Godin Leisure Time Exercise Questionnaire at follow‐up25).
Univariate analysis used to consider associations between demographic and clinical risk factor variables were χ2 (for sex, socioeconomic status, IHD status, blood pressure and cholesterol management, BMI, smoking status and exercise behaviour) and χ2 for trend (for age). Multiple logistic regression was subsequently used to determine which variables independently predicted questionnaire response and consent preference when the effect of other variables was taken into account. Those who had died since baseline and those who were excluded from receipt of the patient questionnaire and consent form were excluded from analyses.
Results
Baseline chart data were available for 1609 patients: 65.4% (n = 1053) male and 34.6% female (n = 556); mean (SD) age was 66 (9.1); 79% were GMS eligible. Patients could be excluded from receipt of the baseline patient questionnaire by their GPs for reasons such as very poor health status or illiteracy. Baseline patient questionnaires were sent to 1577 patients and returned by 1084 (response rate 68.7%).
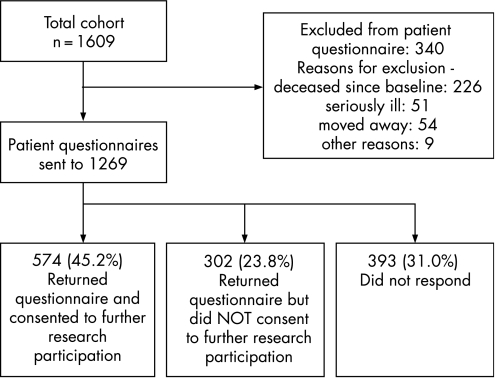
At follow‐up, records were located for 1592 patients (98.9%). The mean (SD) age of the surviving cohort was 69.5 (9.2). Patients could be excluded from receipt of the patient questionnaire at follow‐up for reasons such as death since baseline, serious illness, having moved away from the practice, illiteracy: 340 patients (including all of those for whom no records could be located) were excluded by their GPs for these reasons as outlined in fig 1. Questionnaires were sent to the remaining 1269 patients and were returned by 876 (response rate 69%). Of responders, 574 (65.5%) completed and signed the form which gave consent for participation in further research.
Figure 1 Follow‐up data collection flow chart.
Responders and non‐responders: demographics and risk factors
The levels of non‐response were almost identical between the sexes, with 30.9% of women and 31.0% of men not responding at all. Univariate analysis identified a significant association between response and cholesterol management: of patients whose last serum cholesterol reading was under the recommended 5 mmol/l, 71.8% (n = 522) returned the completed questionnaire compared with 66% (n = 285) of those whose readings were above this level (χ2 = 4.4; df = 1; p<0.05). However, logistic multiple regression identified no variable as independently predictive of questionnaire response.
Consenters and non‐consenters: demographics and risk factors
Univariate analysis
Among responders univariate analysis detected no significant association between consent preferences and either age or socioeconomic status. Neither was any significant association between consent preference and blood pressure management, previous AMI, BMI or exercise behaviour identified. Gender was found to be significantly associated with consent preference, with 68.1% of men (n = 388) compared with 60.8% of women (n = 186) consenting to participation in further research (χ2 = 4.7; df = 1; p<0.05). Of those whose last serum cholesterol reading was below the recommended 5 mmol/l, 69.2% (n = 361) consented to further participation compared with 61.4% (n = 175) of those whose reading was above this level (χ2 = 5; df = 1; p<0.05). Having had surgical cardiac interventions was found to be significantly associated with consent, with 72.5% (n = 150) of those who had had a previous CABG compared with 64% (n = 419) of those who had not consenting (χ2 = 5.1; df = 1; p<0.05) and 74.2% (n = 138) of those who had had PTCA consenting compared with 63.5% (n = 427) of those who had not (χ2 = 7.5; df = 1; p<0.01). Smoking status was associated with consent, with smoking cessation in particular influencing consent preferences: 73.4% (n = 312) of ex‐smokers consented to participation compared with 61.4% (n = 62) of smokers and 61.6% (n = 162) of non‐smokers (χ2 = 13.2; df = 3; p<0.01).
Multiple regression
To evaluate how IHD risk factor variables predicted consent preferences after controlling for other variables, a logistic multiple regression model was fitted to the 540 cases for whom complete data were available, summarised in table 1.
Table 1 Risk factors as predictors of consent to further participation in research: summary of logistic regression (n = 540).
| Risk factors | No (% of total) | Odds ratio (95% CI) |
|---|---|---|
| Consenters | 354 (65.6) | – |
| Gender: male | 359 (66.5) | 1.13 (0.74 to 1.72) |
| Age: increasing | – | 1.000 (0.99 to 1.00) |
| GMS eligible | 397 (73.5) | 0.99 (0.64 to 1.54) |
| Previous AMI | 252 (46.7) | 0.86 (0.59 to 1.26) |
| Previous CABG | 132 (24.4) | 0.98 (0.62 to 1.53) |
| Previous PTCA | 128 (23.7) | 1.77 (1.09 to 2.86)* |
| Last BP <140/90 mm Hg | 379 (70.2) | 1.45 (1.00 to 2.09)* |
| Last total cholesterol <5 mmol/l | 348 (64.4) | 1.71 (1.16 to 2.54)** |
| Ex‐smoker | 283 (52.4) | 1.73 (1.17 to 2.57)** |
| BMI >25 kg/m2 | 365 (67.6) | 0.95 (0.80 to 1.12) |
| Adequate exercise | 291 (53.9) | 1.00 (0.99 to 1.01) |
| Cox and Snell R2 = 0.056; Nagelkerke R2 = 0.078 | ||
AMI, acute myocardial infarction; BMI, body mass index; BP, blood pressure; GMS, General Medical Services; CABG, cardiac artery bypass grafting; PTCA, percutaneous transluminal coronary angioplasty.
*p<0.05; **p<0.01.
Although the explained variation was relatively small, analysis identified four characteristics as independently and significantly positively predictive of consent to further research participation when the effect of other predictors was taken into account: having undergone PTCA, with an odds ratio (OR) of 1.77 (1.09 to 2.86); previous smoking cessation (that is to say being an ex‐smoker rather than a current smoker or non‐smoker), with an OR of 1.73 (1.17 to 2.57); a last recorded blood pressure within recommended levels (<140/90 mm Hg), OR = 1.45 (1.00 to 2.09); and a last recorded total cholesterol level within recommended levels (<5 mmol/l), OR = 1.71 (1.16 to 2.54). Associations were considered among all responders between the variables identified as predictors of consent preference: smoking cessation was significantly associated with PTCA (χ2 = 19.8; df = 1; p<0.01).
What is known on this subject
There is worrying but limited evidence that requiring prior written consent may affect the validity of observational research.
Those with “sensitive” conditions are less likely to consent to the use of their medical data.
In the relatively rare context of intracranial vascular malformation, it has been shown that the requirement for prior consent could affect a study's ability to identify the prognostic importance of a factor which in clinical practice often influences the decision to treat.
The cases included in the analysis represented 61.6% of responders. The loss of 38.4% resulted for the most part from incomplete patient questionnaire data, most notably failure to supply either height or weight, so that BMI could not be calculated. No significant difference in demographic or clinical characteristics was identified between those included and those not included.
What this paper adds
The potential impact of consent bias is demonstrated among a large European community‐dwelling cohort of people with ischaemic heart disease, a disease of everyday clinical importance.
In future, observational research consent bias may mean that determination of the effects of treatments becomes unreliable.
The potential of consent bias to effect the validation of clinically important prognostic predictors is demonstrated in a sample large enough to allow control of other predictors.
Discussion
This study adds to the small but important body of evidence which demonstrates the potential for increasing consent requirements to create selection biases which might undermine observational research. The study has benefited from having access to a large community‐based cohort for whom a rich database based on medical records review and patient questionnaire was available at both baseline and the 5‐year follow‐up. The request for patient's contact details and consent for participation in research was included with patient questionnaires to facilitate subsequent follow‐up, but also afforded the opportunity to consider consent and research participation preferences amongst a large representative cohort of people with IHD.
This study's main finding is that consent bias threatens the validity of future observational research among people with IHD. In this respect, the research supports the findings already published on consent bias. Like Al‐Shahi et al, this research demonstrates that clinically important prognostic variables can be associated with consent preferences, and this has serious implications for future research. However, this study extends these implications from the disease area in the previous research—which is potentially catastrophic but relatively rare—to one which is of everyday clinical importance in Europe.
The size of the cohort in this study allowed sufficient power to conduct multivariate analysis. Unlike existing published results, this research found that although gender was identified as significantly associated with consent preferences by univariate analysis, this was not the case when other predictors were controlled for.
However, the association between clinically important prognostic variables and consent preferences was shown to be significant: patients who had stopped smoking, whose cholesterol and blood pressure were well managed or who had previously benefited from PTCA were more likely to consent to participation in research. Previous PTCA was shown to be associated with smoking cessation. Although it seems likely that the former drives the latter, this cannot be determined from the data; if so, then it might be concluded that it is the healthy lifestyle decision (smoking cessation) rather than the medical intervention which is the true predictor of consent to research participation.
The implication is that if cohorts in the future are dependent upon prior written consent they are likely to contain disproportionate numbers of those who have made healthy lifestyle decisions, who have previously benefited from healthcare or those whose clinical risk factors are already well managed. This may have two serious consequences: first, the generalisability of observational research will be reduced; second, the effects of treatments may be variously overestimated or underestimated if those who are most unwell or are not making healthy lifestyle decisions are under‐represented in study populations.
Policy implications
All the stakeholders in healthcare research, including the public, need to understand and deal with the serious implications for research—and ultimately for medicine—of overzealous interpretation and implementation of confidentiality laws and guidance. The directive approved by the European Parliament which prompted the introduction of new national data protection legislation permitted member states to make exceptions in the case of health‐related research where the benefits to society outweigh any harm attributable to the invasion of privacy.26 In some countries these provisions were not incorporated in legislation so that some forms of observational research have become nearly impossible. In others—such as the UK—the use of identifiable data without prior consent in medical research is permitted in law under certain circumstances. But the associated laws and bureaucracy are complex and public attitudes towards the use of personal medical information in research are unclear. As a result, the requirement for prior consent has emerged as a default position of safety amongst regulatory bodies and research ethics committees.7
If the effectiveness of observational research, of epidemiology and, ultimately, of some elements of medicine are not to be diminished, every effort must be made to ensure that the provisions made available to health‐related research by the European Parliament are adopted and implemented where this is not already the case.
Acknowledgements
We particularly acknowledge the help of the Health Research Board, Noel Scott and Diarmuid O'Donovan of the HSE (Western area) and the practice staff and participating general practitioners: Drs Marcus Allen; Desmond Bluett; Charles Bourke; Sean Bourke; Martin Brennan; Vivian Brennan; James Brogan; Marian Brogan; Declan Clinton; Seamus Cryan; Martin Daly; Anthony Delap; John‐Mark Dick; Ken Egan; Noel Farrell; Mary Feerick; Brendan Forkan; Margaret Gilligan; Enda Harhen; Edward Harty; Richard Joyce; Ciaran Kelly; Bernard McGuire; Pauric Mitchell; Paul Money; Kay Moran; Daniel Murphy; Kieran O'Reilly; John O'Sullivan; Roddy Quinn; John Regan; Michael Regan; Eamonn Shea; John Sheerin; Richard Tobin; David Townley; Kieran Whyte. One GP asked to remain anonymous.
Abbreviations
AMI - acute myocardial infarction
BMI - body mass index
CABG - cardiac artery bypass grafting
GMS - General Medical Services
GP - general practitioner
IHD - ischaemic heart disease
OR - odds ratio
PTCA - percutaneous transluminal coronary angioplasty
Footnotes
Funding: Baseline and follow‐up studies were both funded by the Health Research Board, Dublin and the Health Services Executive (Western Area).
Conflict of interest: None.
References
- 1.Medical Research Council Personal information in medical research. London: Medical Research Council, 2000
- 2.Anonymous An information guide to the data proctection acts for general practitioners. Irish College of General Practitioners/National General Practice Information Technology Group 2003
- 3.Data Protection Commissioner Data Protection Acts 1988 and 2003: a compendium. Dublin: Office of the Data Protection Commissioner, 2003
- 4.Data Protection Commissioner Data Protection (Amendment) Act 2003: a summary guide. Dublin: The Office of the Data Protection Commissioner, 2003
- 5.Anonymous Health information: a national strategy. Dublin: Department of Health and Children, 2004
- 6.Department of Health Research governance framework for health and social care. 2nd ed. London: Department of Health, 2005
- 7.Academy of Medical Sciences Personal data for public good: using health information in medical research. London: The Academy of Medical Sciences, 2006, Available at http://www.acmedsci.ac.uk (accessed 3 June 2007)
- 8.Kalra D, Gertz R, Singleton P.et al Confidentiality of personal health information used for research. BMJ 2006333196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robling M R, Hood K, Houston H.et al Public attitudes towards the use of primary care patient record data in medical research without consent: a qualitative study. J Med Ethics 200430104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singleton P, Wadsworth M. Consent for the use of personal medical data in research. BMJ 2006333255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone M A, Redsell S A, Ling J T.et al Sharing patient data: competing demands of privacy, trust and research in primary care. Br J Gen Pract 200555783–789. [PMC free article] [PubMed] [Google Scholar]
- 12.Baker R, Shiels C, Stevenson K.et al What proportion of patients refuse consent to data collection from their records for research purposes? Br J Gen Pract 200050655–656. [PMC free article] [PubMed] [Google Scholar]
- 13.Willison D J, Keshavjee K, Nair K.et al Patients' consent preferences for research uses of information in electronic medical records: interview and survey data. BMJ 2003326373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cousins G, McGee H, Ring L.et alPublic perceptions of biomedical research: a survey of the general population in Ireland. Dublin: Health Research Board, 2005
- 15.Al‐Shahi R, Vousden C, Warlow C, for the Scottish Intracranial Vascular Malformation Study (SIVMS) Steering Committee et al Bias from requiring explicit consent from all participants in observational research: prospective, population based study. BMJ 2005331942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al‐Shahi R, Warlow C. Using patient‐identifiable data for observational research and audit. BMJ 20003211031–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson A J. Commentary: Methodological reasons for not gaining prior informed consent are sometimes justified. BMJ 200432987–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobsen S J, Xia Z, Campion M E.et al Potential effect of authorization bias on medical record research. Mayo Clin Proc 199974330–338. [DOI] [PubMed] [Google Scholar]
- 19.Regidor E. The use of personal data from medical records and biological materials: ethical perspectives and the basis for legal restrictions in health research. Soc Sci Med 2004591975–1984. [DOI] [PubMed] [Google Scholar]
- 20.Tu J V, Willison D J, Silver F L.et al Impracticability of informed consent in the registry of the Canadian stroke network. N Engl J Med 20043501414–1421. [DOI] [PubMed] [Google Scholar]
- 21.Angus V C, Entwistle V A, Emslie M J.et al The requirement for prior consent to participate on survey response rates: a population‐based survey in Grampian. BMC Health Serv Res 2003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrne M, Murphy A W, Walsh J C.et al A cross‐sectional study of secondary cardiac care in general practice: impact of personal and practice characteristics. Fam Pract 200623295–302. [DOI] [PubMed] [Google Scholar]
- 23.WHO Diet, nutrition and prevention of chronic disease. Report of a WHO study group. WHO Technical Report Series 797. Geneva: World Health Organisation, 1990 [PubMed]
- 24.Wood D, De Backer G, Faergeman O.et al Prevention of coronary heart disease in clinical practice: recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. Eur Heart J 1998191434–1503. [DOI] [PubMed] [Google Scholar]
- 25.Godin G, Shephard R J. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 198510141–146. [PubMed] [Google Scholar]
- 26.European Community Directive 95/46/EC of the European Parliament and of the Council of 24 October 1995 on the protection of individuals with regard to the processing of personal data and on the free movement of such data. Official Journal of the European Communities 1995L31–35. [Google Scholar]