Abstract
Background
In the Padova International Classification, gastric precancerous lesions are labelled as “indefinite for non‐invasive neoplasia” (Indef‐NiN) cytohistological alterations mimicking non‐invasive neoplasia (NiN), but lacking all the attributes required for a definite NiN categorisation.
Aim
To apply a panel of immunohistochemical (IHC) markers of cell proliferation (Mib1), intestinal differentiation (Cdx2), apoptosis (pro‐caspase 3) and cell immortalisation (hTERT) to compare the IHC profiles of a series of precancerous lesions arising in gastric intestinalised (ie, IM‐positive) glands.
Materials and methods
By applying the histological criteria consistently provided by both the Padova Classification and the World Health Organization International Agency, 112 consecutive cases were considered: intestinal metaplasia (IM; n = 54), Indef‐NiN in IM‐positive gastric glands (n = 28) and low‐grade (LG) NiN (n = 30). In each histological category, the expression of the marker was separately scored in superficial, proliferative and coil compartments.
Results
In all glandular compartments, Mib1, Cdx2, hTERT and pro‐caspase 3 were consistently more expressed in LG‐NiN than in either IM or Indef‐NiN lesions (analysis of variance: p<0.001). Significant ORs (calculated by ordinal logistic regression analysis for each glandular compartment) associated IM, Indef‐NiN and LG‐NiN with the expression of the considered markers.
Conclusions
A consistent overexpression (unrestricted to the proliferative zone) of IHC markers of cell proliferation, intestinal differentiation, decreased apoptosis and cell immortalisation differentiates LG‐NiN from both (simple) IM and Indef‐NiN (arising in IM). An increased proliferative activity in the proliferative zone discriminates Indef‐NiN lesions (ie, hyperproliferative IM) from IM. Such divergent IHC profiles may provide a rationale for scheduling follow‐up protocols more properly tailored on the patient's risk for cancer.
The World Health Organization International Agency has recently redefined dysplasia as intraepithelial (ie, non‐invasive) neoplasia (NiN), and this definition is consistent with that proposed by the International Padova Classification for gastric precancerous lesions.1,2 As in other epithelial areas, the biological profile of gastric NiN shares significant molecular attributes with invasive cancer, but lacks any evidence of invasion (of neoplastic cells) into the surrounding stroma.1,2 Consistently, both the World Health Organization Agency and the Padova proposal define those phenotypic alterations in which pathologists cannot discriminate between hyperplastic and true preneoplastic lesions as “indefinite for NiN” (Indef‐NiN). 1,2 According to this definition, the diagnosis of Indef‐NiN covers a grey zone of phenotypic alterations lacking some of the histological features required for a definite diagnosis of NiN. The histological distinction between Indef‐NiN lesions and definite low‐grade (LG) NiN is characterised by a low–moderate inter‐observer consistency, which may result in inconsistent guidelines for clinicoendoscopic follow‐up.1,2
Gastric Indef‐NiN lesions consist of LG‐cytoarchitectural abnormalities, which tend to decrease from the base of the glands to their superficial portion, and may coexist with high‐grade inflammation.1,2 Two variants of Indef‐NiN lesions are described: (a) foveolar hyperproliferation and (b) hyperproliferative intestinal metaplasia (IM).2 Foveolar hyperproliferation affects native (ie, non‐metaplastic) gastric glands and often coexists with erosion/ulcer; hyperproliferative IM mostly consists of demarcated foci of intestinalised glands, with a back‐to‐back architecture and a moderate‐to‐high mitotic rate. Hyperproliferative IM is more commonly encountered in diagnostic routine than in foveolar hyperproliferation. Long‐term follow‐up studies demonstrated that Indef‐NiN rarely progresses to more severe lesions, but, because of the lack of consistent criteria of histological distinction, Indef‐NiN and LG NiN lesions are prudentially managed according to similar follow‐up protocols.3,4,5
Although no molecular markers are available to discriminate between non‐neoplastic and neoplastic non‐invasive lesions, increased cell turnover, cell dedifferentiation, cell immortalisation and apoptosis deregulation are all associated more strictly with neoplastic than with non‐neoplastic cell growth. IHC markers are available for each of the above biological properties and have been internationally validated.6,7,8,9,10,11,12,13,14,15,16,17,18,19,20
This retrospective cross‐sectional study is aimed to characterise the biological profile of Indef‐NiN lesions originating from gastric intestinalised glands. For this purpose, a panel of IHC markers of cell turnover (Mib1),6,7,8 cell differentiation (Cdx2),9,10,11 cell immortalisation (hTERT)12,13,14,15 and apoptosis (pro‐caspase 3)16,17,18,19,20 was used to compare the immunophenotype of Indef‐NiN arising in IM‐positive gastric epithelia with the IHC profiles of a series of truly non‐neoplastic lesions (ie, gastric IM) and definitely neoplastic non‐invasive lesions (LG NiN).
Patients and methods
Patients
The cases in the present study were retrospectively collected by selecting consecutive cases of gastric IM, Indef‐NiN and LG NiN lesions, all histologically assessed at the Department of Pathology, Padova University, Padova, Italy, between July 2003 and June 2004. All patients had undergone upper gastrointestinal endoscopy for dyspepsia. The patients' history was established from the clinical information obtained at the time of the endoscopy. Patients with inconsistent clinical history, and/or who had received anti‐Helicobacter pylori antibiotic treatment, and/or were taking proton pomp inhibitors, and/or had previously had surgical/endoscopic treatment (partial gastrectomy or mucosectomy), were ruled out. According to the clinical information available at the time of the endoscopy procedure, 6 out of 112 (5%) patients were taking non‐steroidal anti‐inflammatory drugs (NSAIDs); in none of these patients was mucosal alteration endoscopically detected. At least five biopsy samples were obtained according to the Updated Sydney System's biopsy sampling protocol (two corpus, two antrum and one incisura angularis sample).21,22 However, for the purposes of the present study, only the biopsy sample showing the target phenotypic alteration was considered. H pylori infection was assessed on the whole available biopsy set.
Cases whose biopsy specimens were not well oriented and/or did not include full‐thickness mucosa (representative of the muscularis mucosae) were also excluded. Only biopsy samples obtained from antral or incisural mucosa were considered. According to the said criteria, 112 consecutive cases were included: (a) 54 cases of gastric IM; (b) 28 cases of Indef‐NiN (all of them arising in intestinalised glands); and (c) 30 cases of LG NiN (all of them coexisting with IM).
Table 1 shows the demographics and clinicopathological characteristics of each group of patients (including H pylori status, clinical history of NSAID use and the mucosal area from where the target biopsy sample was obtained).
Table 1 Patient's demographics, Helicobacter pylori status and of clinical history of use of non‐steroidal anti‐inflammatory drugs.
| Histological category | Cases | M/F | Age: mean/median (range); years | Helocobactor pylori‐positive patients (%) | NSAIDs (%) | Location | Gastritis OLEA‐stage | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antrum | Angularis incisura | 0 | I | II | III | IV | ||||||
| IM | 54 | 20/34 | 63.7/65.5 (33–87) | 36 (67) | 3 (5.5) | 26 | 28 | 0 | 1 | 18 | 22 | 13 |
| Indef‐NiN | 28 | 18/10 | 61.6/62.0 (28–88) | 23 (82) | 2 (7.1) | 15 | 13 | 0 | 0 | 5 | 15 | 8 |
| LG‐NiN | 30 | 17/13 | 62.4/62.5 (41–80) | 25 (83) | 1 (3.3) | 17 | 13 | 0 | 0 | 1 | 16 | 13 |
F, female; M, male; NiN, non‐invasive neoplasia; NSAID, non‐steroidal anti‐inflammatory drug; IM, intestinal metaplasia; Indef‐NiN, indefinite for NiN; LG‐NiN, low‐grade NiN.
The gastric location (antrum and angularis incisura) from where the considered biopsy specimen was obtained is also specified.
IM group
A total of 54 consecutive cases of IM were considered (M/F: 20/34; mean (range) age 63.7 (33–87) years). Among all the biopsy specimens considered, the presence of goblet cells was confirmed (Alcian‐PAS) in >50% of gastric glands (IM score 2/3, according to the Gentas' visual analogue scale).22
Indef‐NiN group
A total of 28 (M/F: 18/10; mean (range) age 61.6 (28–88) years) Indef‐NiN cases were selected. The histological criteria adopted for their assessment have been extensively explained elsewhere.1,2 In the present series, only consecutive cases of Indef‐NiN lesions arising in intestinalised glands (category 2.2, according to the Padova International classification) were included. The only one case of Indef‐NiN arising in non‐metaplastic glands (ie, foveolar hyperproliferation type; category 2.1, according to the Padova International Classification), encountered between July 2003 and June 2004, was detected in a superficial biopsy sample obtained from a patient using NSAIDs. The biopsy specimen did not include full‐thickness mucosa and was not considered suitable for this study.
LG‐NiN group
A total of 30 consecutive cases of LG‐NiN (M/F: 17/13; mean (range) age 62.4 (41–80) years) were included. According to the current criteria, the classification of LG‐NiN strictly demanded an LG of both glandular architecture and abnormal cytological differentiation, but with no (even dubious) infiltrating features.1,2
Histological study
All biopsy samples were fixed in 10% buffered formalin and embedded in paraffin wax. Serial histological sections of 5 μm thickness were obtained from each selected paraffin wax block. The histological sections were stained with H&E, Alcian blue and Giemsa modified for H pylori. According to the Updated Sydney System, granulocyte infiltrate (intraepithelial and/or within the lamina propria) was semiquantitatively scored in a four‐tiered scale ranging from 0 = absent to 3 = marked, on the Gentas' visual analogue scale.22 The same scale was used to score the mononuclear infiltrate within the lamina propria. The stage of the gastritis was assessed according to the Operative Link for Gastritis Assessment's International criteria (OLEA‐staging).23,24H pylori was scored as present or absent.
After collecting the archival series, the original diagnosis was validated by histologically re‐evaluating all cases. The interobserver agreement in the histological assessment of the three histological categories was tested using k statistics for pairs of observers (two experienced pathologists (MC and MR)). Cases inconsistently classified were jointly reconsidered for the final histological assessment.
Immunohistochemical methods and labelling index assessment
For the IHC study, serial sections (5/6 μm thick) were obtained. Sections were dewaxed with xylene, rinsed in absolute alcohol, treated with 3% hydrogen peroxide (5 min), and then rinsed in phosphate‐buffered saline (PBS; three times, 5 min each). The antigen retrieval protocol was performed in a microwave oven, placing the sections in citrate buffer, pH 6 (microwave protocol consisted of six distinct steps (20 s each), at a progressively declining power from 500 to 160 W). After performing the retrieval protocol, the sections were incubated (30 min at room temperature), with protein blocking Agent UltraTech HRP kit (Immunotech; Marseille, France). Sections were stained with primary antibody (working dilution, temperature and incubation time are reported for each antibody). After washing in PBS, the sections were incubated with a biotinylated secondary antibody (Ultratech HRP kit, Marseille, France; 10 min at room temperature). The sections were then incubated with streptavidin–peroxidase reagent (Ultratech HRP kit; 10 min at room temperature) and, after washing in PBS, the reaction was developed in DAB Substrate‐Chromogen System (Dako‐Cytomation, Carpinteria, California, USA) for a few seconds. The sections were washed in water, counterstained with Mayers' haematoxylin, dried in alcohol, cleared in xylene and mounted.
The following antigens were tested:
Mib1 for human ki‐67 antigen (monoclonal antibody; anti‐MIB‐1 clone code n. M7240, DAKO, Glostrup, Denmark; working dilution 1:50; incubation time: 60 min at room temperature);
Cdx2 antigen of intestinal differentiation (monoclonal antibody anti‐Cdx2 AMT28 clone, Novocastra Laboratories, Newcastle Upon Tyne, UK; working dilution 1:200; incubation time: 60 min at room temperature);
hTERT antigen of catalytic protein subunit of human telomerase (monoclonal antibody anti‐hTERT 4F12 clone, Novocastra Laboratories; 1:30 dilution; incubation time: 60 min at room temperature);
pro‐caspase 3 (ie, CPP32‐His6) human antigen (monoclonal antibody anti‐CPP32‐His6 3CSP03 (same as 4.1.18) human antigen LAB VISION Corporation‐NeoMarkers, California, USA; working dilution 1:75; incubation time: 60 min at room temperature).
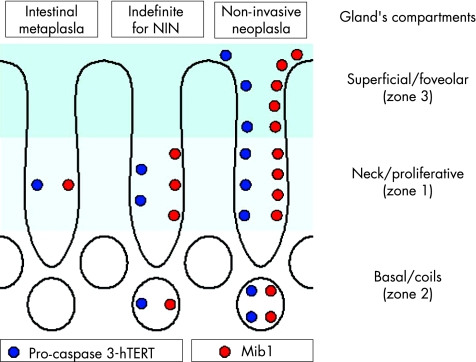
Immunohistochemical staining was semiquantitatively scored in each of the three functional compartments of the gastric glands, distinguishing: (a) proliferative (zone 1), (b) coiled (zone 2) and (c) foveolar/superficial (zone 3) compartments.25 In each zone, the positive immunostain (nuclear or cytoplasmic) was scored as a percentage in at least 300 cells: 0 = no positive stain; 1 = positive stain in <30% of the cells; 2 = positive stain in 31–60%; and 3 = positive stain in >60% of the cells.
Statistical analysis
The t test and one‐way analysis of variance (ANOVA) were applied for statistical calculations. Odds ratios (ORs) and 95% CIs were calculated to assess the strength of association between variables, using the ordinal logistic regression model (OR 1 indicating no association). STATA software (Statistics Data Analysis, V.8.1) was used for all calculations. A p value of <0.05 was considered significant.
The inter‐observer agreement for pairs of observers was calculated using k statistics. The k coefficient was interpreted in accordance with the benchmarks proposed by Landis and Koch (<0.4 = poor agreement; 0.41–0.8 = moderate/good agreement; >0.8 = excellent agreement).26
Results
Inter‐observer agreement for histological categories
K coefficients for the histological categorisation of each of the lesions considered were as follows: intestinal metaplasia: 0.90; Indef‐ NiN: 0.75; LG NiN: 0.81.
Histological scores of inflammation and gastritis staging
Mononuclear infiltrate in the lamina propria was lowest in IM (mean (SD) score 1.44 (0.57)) and highest in Indef‐NiN (mean (SD) score 1.78 (0.49)); LG‐NiN (mean (SD) score 1.5 (0.5)) featured an intermediate mean value (ANOVA; p = 0.02). Mononuclear infiltrate density differed significantly when comparing IM with Indef‐NiN, and Indef‐NiN with LG‐NiN (t test; p<0.006 and p<0.035, respectively).
Granulocyte infiltrate in the lamina propria and within glands did not differ in the three histological categories.
Table 1 shows the stage of the gastritis associated with each of the considered lesions.
Immunohistochemical study
Mib1
Tested by ANOVA, expression of Mib1 (in all glandular compartments) differed significantly in IM, Indef‐NiN and LG‐NiN (p<0.001).
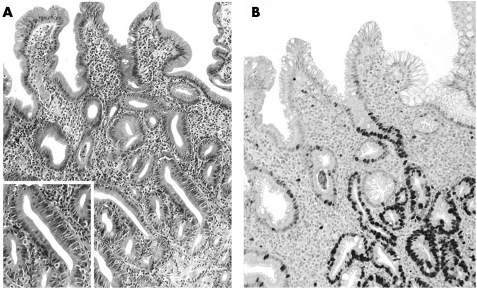
In IM cases, expression of Mib1 was never detected in the superficial/foveolar zone; the difference in marker expression was significant only for Indef‐NiN when compared with LG‐NiN (t test; p<0.001;figs 1 and 2; table 2).
Figure 1 (A,B) Indefinite for non‐invasive neoplasia: Helicobacter pylori‐associated hyperproliferative intestinal metaplasia. H&E stain (A) shows crowded, intestinalised glands coexisting with “mature” epithelia in the superficial layer; Mib1 expression (B) (absent in the superficial compartment) is evident from the proliferative zone to the base of the glandular structures (original magnification: ×40).
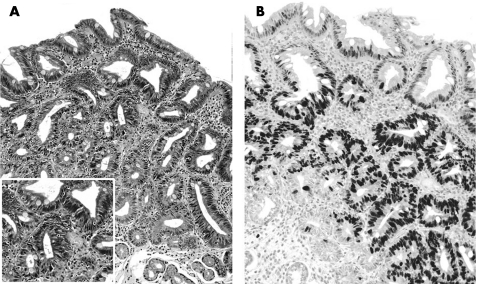
Figure 2 (A–B) Low‐grade non‐invasive neoplasia (NiN): H&E stain (A) shows all the spectrum of the cytohistological lesions of the NiN (altered architecture, atypical/pseudostratified nuclei, loss of superficial differentiation). (B) Mib1 nuclear immunostain is clearly demonstrated throughout the glandular units (from the superficial to the “basal” epithelia; original magnification: ×40).
Table 2 Expression of Mib1, Cdx2, hTERT and pro‐caspase 3 in the three glandular zones (superficial/foveolar, proliferative and coiled portions) in the different histological categories.
| Marker expression according to glandular compartment | IM | t Test p value IM vs Indef‐NiN | Indef‐NiN | t Test p value Indef‐NiN vs LG‐NiN | LG‐NiN | ANOVA p value IM vs Indef‐NiN vs LG‐NiN |
|---|---|---|---|---|---|---|
| Mib1 | ||||||
| Superficial/foveolar zone | 0 | NS | 0.17 (0.61) | <0.001 | 1.37 (1.2) | <0.001 |
| Proliferative zone | 1.61 (0.62) | <0.001 | 2.39 (0.49) | NS | 2.48 (0.5) | <0.001 |
| Glandular coils | 0.05 (0.23) | NS | 0.14 (0.52) | 0.002 | 1.17 (1.25) | <0.001 |
| Cdx2 | ||||||
| Superficial/foveolar zone | 0.27 (0.65) | NS | 0.26 (0.53) | <0.001 | 1.58 (1.18) | <0.001 |
| Proliferative zone | 1.7 (0.66) | <0.001 | 2.42 (0.5) | 0.048 | 2.65 (0.48) | <0.001 |
| Glandular coils | 0.12 (0.47) | 0.035 | 0.5 (0.86) | <0.001 | 1.68 (1.36) | <0.001 |
| hTERT | ||||||
| Superficial/foveolar zone | 0 | NS | 0.07 (0.26) | <0.001 | 0.68 (0.74) | <0.001 |
| Proliferative zone | 0.81 (0.62) | <0.001 | 1.59 (0.69) | NS | 1.8 (0.57) | <0.001 |
| Glandular coils | 0.03 (0.47) | 0.02 | 0.33 (0.62) | NS | 0.52 (0.71) | <0.001 |
| Pro‐caspase 3 | ||||||
| Superficial/foveolar zone | 0.07 (0.33) | <0.001 | 0.5 (0.57) | 0.006 | 1.03 (0.89) | <0.001 |
| Proliferative zone | 1.28 (0.60) | <0.001 | 1.85 (0.52) | NS | 1.88 (0.97) | <0.001 |
| Glandular coils | 0.35 (0.52) | 0.039 | 0.64 (0.62) | NS | 0.74 (0.94) | <0.001 |
ANOVA, analysis of variance; Indef‐NiN, indefinite non‐invasive neoplasia; IM, intestinal metaplasia; LG‐NiN, low‐grade non‐invasive neoplasia; NS, not significant.
In all the diagnostic categories, the proliferative zone was associated with the highest Mib1 expression; a significant difference emerged when comparing Mib1 expression in IM with that in Indef‐NiN (t test; p<0.001; table 2).
In glandular coils, a significantly higher score was associated with LG‐NiN compared with Indef‐NiN lesions (t test; p<0.002), whereas no difference was seen for IM when compared with Indef‐NiN (t test; p = NS; table 2).
By distinguishing the three glandular compartments, significant ORs (p<0.001) associated Mib1 expression with the three histological categories (zone 3: OR 8.8; 95% CI 3.3 to 23.0; zone 1: OR 7.4; 95% CI 3.6 to 15.0; zone 2: OR 4.4; 95% CI 2.3 to 8.5 (table 3)).
Table 3 ORs and 95% CI for the expression of Mib1, Cdx2, hTERT and pro‐caspase 3 in the three glandular compartments, according to the dependent ordinal variables considered.
| Glandular compartment | Mib1 | Cdx2 | hTERT | Pro‐caspase 3 |
|---|---|---|---|---|
| Superficial/foveolar (zone 3) | 8.8 (3.3 to 23.0) | 2.3 (2.0 to 5.2) | 33.1 (6.7 to 163.9) | 7.8 (3.6 to 16.8) |
| Proliferative (zone 1) | 7.4 (3.6 to 15.0) | 8.6 (4.1 to 18.0) | 8.8 (3.0 to 11.2) | 3.2 (1.8 to 5.7) |
| Coils (zone 2) | 4.4 (2.3 to 8.5) | 3.3 (2.1 to 5.0) | 4.1 (1.9 to 8.8) | 2.0 (1.1 to 3.4) |
Values are given as OR (95% CI).
For all but one (Cdx2 in glandular coils 2 = p<0.01), the reported ORs were significant at a p value of <0.001.
Dependent ordinal variables considered were intestinal metaplasia, indefinite for non‐invasive neoplasia and low‐grade non‐invasive neoplasia.
Cdx2
Consistently with the presence of IM (H&E stain), a positive immunoreaction was demonstrated in all cases. The glandular compartments showed a significantly different expression of Cdx2 in the three diagnostic categories (IM, Indef‐NiN and LG‐NiN: ANOVA; p<0.001). Overall, Cdx2 expression progressively increased in each glandular compartment, from IM to Indef‐NiN to LG‐NiN (table 2).
No difference was detected in Cdx2 expression in the superficial compartment when comparing IM with Indef‐NiN cases, whereas Cdx2 expression significantly differentiated Indef‐NiN from LG‐NiN in this zone (t test; p = 0.001; table 2).
The proliferative zone showed significant differences in marker expression in both comparisons—that is, IM versus Indef‐NiN, and Indef‐NiN versus LG‐NiN (t test; p<0.001 and p<0.048, respectively; table 2).
A marginally significant difference emerged when comparing coil Cdx2 expression in IM with that in Indef‐NiN (t test, p = 0.035); the different expression of Cdx2 significantly discriminated between Indef‐NiN and LG‐NiN (t test; p = 0.001; table 2).
Table 3 shows the ORs for associations between Cdx2 expression and the three histological categories. In each glandular compartment, significantly different ORs associated Cdx2 expression with the three histological categories (ordinal logistic regression analysis; zone 3: OR 2.3, 95% CI 2.0 to 5.2 (p<0.01); zone 1: OR 8.6, 95% CI 4.1 to 18.0 (p<0.001); zone 2: OR 3.3, 95% CI 2.1 to 5.0 (p<0.001)).
hTERT
In all glandular compartments, expression of hTERT increased from IM to Indef‐NiN to LG‐NiN (ANOVA; p<0.001; table 2). The proliferative zone showed the highest expression of hTERT in all histological categories. IM cases never showed any expression of hTERT in the superficial/foveolar compartment.
Significant differences in marker expression were disclosed by comparing IM with Indef‐NiN (proliferative zone), and Indef‐NiN with LG‐NiN (superficial/foveolar zone; t test: p<0.001; table 2; fig 3).
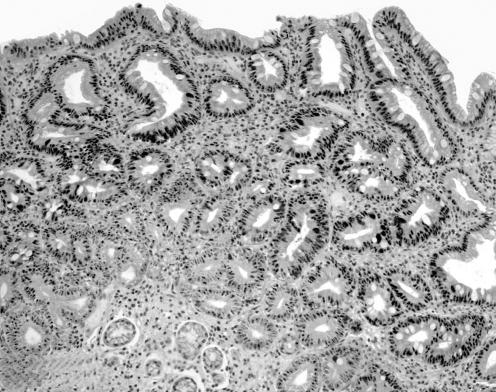
Figure 3 Low‐grade non‐invasive neoplasia: nuclear immunostain for hTERT(2b) is clearly demonstrated all throughout the glandular units (from the superficial to the “basal” epithelia; original magnification: ×20).
Significant ORs associated hTERT expression (in each glandular compartment) with the three histological categories (ordinal logistic regression analysis; zone 3: OR 33.1, 95% CI 6.7 to 163.9 (p<0.001); zone 1: OR 8.8, 95% CI 3.0 to 11.2 (p<0.001); zone 2: OR 4.1, 95% CI 1.9 to 8.8 (p<0.001); table 3).
Pro‐caspase 3
In all three glandular compartments, expression of pro‐caspase 3 increased progressively from IM to Indef‐NiN to LG‐NiN (ANOVA; p<0.03). In all groups, the proliferative zone showed the highest expression of pro‐caspase 3 (table 2).
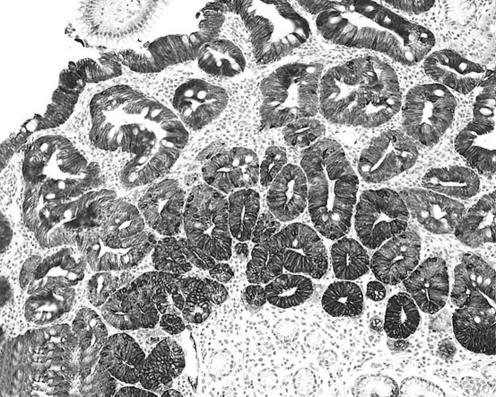
In the superficial/foveolar compartment, expression of pro‐caspase 3 significantly discriminated between IM and Indef‐NiN lesions, and between Indef‐NiN and LG‐NiN (t test; p<0.001 and p<0.006, respectively; table 2; fig 4).
Figure 4 Low‐grade non‐invasive neoplasia: cytoplasm and nuclei immunoreaction for pro‐caspase 3; the non‐invasive neoplastic lesion exhibits immunostain from the top to the base of the glandular structures (original magnification: ×20).
In both proliferative and coiled zones, a significant difference emerged when the expression of pro‐caspase 3 was compared in IM versus Indef‐NiN lesions (t test; p<0.001 and p<0.039, respectively). No difference in marker expression was detected between these two zones in the Indef‐NiN versus LG‐NiN cases (table 2).
Table 3 shows the ORs for the association between pro‐caspase 3 expression and histological categories (ordinal logistic regression analysis; zone 3: OR 7.8, 95% CI 3.6 to 16.8 (p<0.001); zone 1: OR 3.2, 95% CI 1.8 to 5.7 (p<0.001); zone 2: OR 2.0, 95% CI 1.1 to 3.4 (p<0.001); table 3).
H pylori status and NSAID assumption versus marker expression
The score values of Mib1, Cdx2, hTERT and pro‐caspase 3 expression were distinguished according to the H pylori status. The marker expression associated with each of the glandular zones was compared in IM versus Indef‐NiN, and versus LG‐NiN cases. No significant differences were detected in any of the glandular compartments for the expression of Cdx2, hTERT and pro‐caspase 3. Only in IM cases was a marginally significant increased expression of Mib1 associated with the proliferative zone of H pylori‐positive cases (Mib1 expression in the proliferative zone of H pylori‐positive versus Mib1 expression in the proliferative zone of H pylori‐negative cases; p = 0.058).
Owing to the low number of patients using NSAIDs, no significant differences were disclosed in marker expression, based on the considered clinical variable.
Discussion
This study explored the IHC expression of a panel of biological markers in different precancerous lesions (IM, Indef‐NiN and LG‐NiN) involved in the spectrum of multistep gastric carcinogenesis.1,2,27
Histology demonstrated a significantly higher score for inflammatory cells within the lamina propria in Indef‐NiN lesions than in cases with both LG‐NiN and IM: this is consistent with the assumption that Indef‐NiN lesions are most commonly encountered in association with (or promoted by) mucosal inflammation.1,2
Overall, the results of the IHC study consistently associated LG‐NiN with a higher proliferative activity, a more extensive glandular intestinalisation and a greater tendency for cell immortalisation than in the case of either IM or Indef‐NiN. Such an IHC profile is consistent with the current hypothesis on the neoplastic nature of gastric dysplasia, and further supports its categorisation as NiN.1,2
In all glandular compartments, expression of Cdx2 was significantly associated with LG‐NiN. This reliable top–down overexpression of the intestinalisation marker differentiated NiN from both IM and Indef‐NiN, providing evidence of a definitely (metaplastic) intestinal commitment associated with NiN. Such a consolidated intestinal phenotype is consistent with the “field cancerisation process” leading to the development of intestinal‐type gastric cancer.9,28,29
The results of this study disclosed a divergent expression of the IHC markers considered in the different compartments of the gastric glands of each of the histological categories.
As expected, the gland's proliferative zone (where glandular stem cells normally lie) was characterised by the high‐level concurrent expression of the markers associated with cell proliferation and immortalisation (with a low tendency to apoptosis).13,15,30 It is noteworthy, however, that Indef‐NiN cases were associated with a significantly higher proliferative activity (and lower tendency to apoptosis) than that detected in IM cases. Such an IHC profile is consistent with the pseudoadenomatous growth pattern (back‐to‐back glands, high proliferative rate), generating a confounding phenotype in its distinction with LG‐NiN.1,2,31
Significant information on the divergent biological profile of IM, Indef‐NiN and LG‐NiN was obtained by selectively scoring both the superficial and the basal glandular zones. To be more specific, LG‐NiN was characterised by a consistent overexpression of Mib1 and hTERT in both superficial and basal zones (where the markers were significantly less expressed in both Indef‐NiN and IM; fig 5). This unrestricted cell proliferation/immortaliation (Mib1/hTERT) is consistent with the neoplastic nature of NiN.30 The diagnostic value of such an IHC pattern was further supported by the pro‐caspase 3 (ie, CPP32‐His6 = inactive caspase) overexpression in the same glandular zones. The accumulation/segregation of CPP32‐His6 in the superficial compartment further characterised the population of the superficial cells as being more prone to proliferation than to apoptosis, a propensity that is an adjunctive attribute of any neoplastic growth.17,19,20
Figure 5 Visual analogue scale showing the divergent expression of the immunohistochemical markers in intestinal metaplasia, indefinite for non‐invasive neoplasia (NIN) lesions and low‐gradea.
The IHC profile of Indef‐NiN lesions and IM supports a significant biological similarity between the two lesions, and also shows their divergent proliferative aptitude. In fact, the two lesions share the same negligible expression of Mib1, hTERT and pro‐caspase 3 in the superficial compartment (as expected for a non‐neoplastic cell growth), but a significantly higher proliferative index in the proliferative zone differentiates Indef‐NiN lesions from typical IM. This biological profile qualifies Indef‐NiN lesions as a variant of gastric gland intestinalisation prone to exaggerated cell proliferation.31 As a consequence, the definition of hyperproliferative IM (category number 2.2 in the Padova International Classification) seems to be more appropriate than the diagnostic label of Indef‐NiN, since the latter leads to three main undesirable consequences: an indefinite message for the clinician, excessive anxiety for the patient, and the latter's unwarranted inclusion in a category at high risk of gastric cancer.2
If it is validated by prospective follow‐up studies, this biological categorisation and labelling of gastric lesions in Indef‐NiN might locate hyperproliferative IM more appropriately within the spectrum of gastric oncogenic processes, also providing a solid rationale for the patient's follow‐up.32,33,34
Take‐home messages
In gastric precancerous lesions, indefinite for non‐invasive neoplasia (Indef‐NiN) alterations show cytohistological abnormalities that mimic non‐invasive neoplasia (NiN) but lack all the attributes required for a definite NiN categorisation.
Distinction of Indef‐NiN lesions from both simple gastric intestinalisation and low‐grade NiN is crucial in scheduling the patient's follow‐up.
By applying an immunohistochemical panel of antibodies exploring: (a) cell proliferation (Mib1), (b) intestinal differentiation (Cdx2), (c) apoptosis (pro‐caspase 3) and (d) cell immortalisation (hTERT), Indef‐NiN lesions may be consistently distinguished from both simple intestinal metaplasia and low‐grade NiN.
Acknowledgements
Quality control on the immunohistochemical tests was carried out by Dr C Lanza, Dr E Portolan and Dr P Bettonte.
Abbreviations
ANOVA - analysis of variance
IHC - immunohistochemical
IM - intestinal metaplasia
Indef‐NiN - indefinite for non‐invasive neoplasia
LG - low grade
NiN - non‐invasive neoplasia
NSAID - non‐steroidal anti‐inflammatory drug
PBS - phosphate‐buffered saline
Footnotes
Funding: This study was supported by the Oncology Institute of the Veneto Region (IOV‐IRCCS), the Italian Association for Cancer Research (AIRC) and the Farini Association for Gastroenterological Research.
Competing interests: None declared.
References
- 1.Fenoglio‐Preiser C, Carneiro F, Correa P.et al Gastric carcinoma. In: Hamilton SR, Aaltonen LA, eds. Pathology and genetics, tumors of the digestive system. Lyon, France: IARC Press, 200039–52.
- 2.Rugge M, Correa P, Dixon M F.et al Gastric dysplasia: the Padova International Classification. Am J Surg Pathol 200024167–176. [DOI] [PubMed] [Google Scholar]
- 3.Rugge M, Leandro G, Farinati F.et al Gastric epithelial dysplasia. How clinicopathologic background relates to management. Cancer 199576376–382. [DOI] [PubMed] [Google Scholar]
- 4.Rugge M, Farinati F, Baffa R.et al Gastric epithelial dysplasia in the natural history of gastric cancer: a multicenter prospective follow‐up study. Interdisciplinary Group on Gastric Epithelial Dysplasia. Gastroenterology 19941071288–1296. [DOI] [PubMed] [Google Scholar]
- 5.Rugge M, Cassaro M, Di Mario F.et al The long term outcome of gastric non‐invasive neoplasia. Gut 2003521111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Igarashi N, Takahashi M, Ohkubo H.et al Predictive value of Ki‐67, p53 protein and DNA content in the diagnosis of gastric carcinoma. Cancer 1999861449–1454. [DOI] [PubMed] [Google Scholar]
- 7.Shipper D, Wagenmans M J M, Peters W H M.et al Significance of cell proliferation measurement in gastric cancer. Eur J Cancer 199834781–790. [DOI] [PubMed] [Google Scholar]
- 8.Testino G, Gada D, De Iaco F.et al p53 and Ki‐67 expression in epithelial gastric dysplasia and in gastric cancer. Panminerva Med 200244369–371. [PubMed] [Google Scholar]
- 9.Silberg D G, Swain G P, Suh E R.et al Cdx1 and Cdx2 expression during intestinal development. Gastroenterology 2000119961–971. [DOI] [PubMed] [Google Scholar]
- 10.Seno H, Oshima M, Taniguchi M A.et al Cdx2 expression in the stomach with intestinal metaplasia and intestinal‐type cancer: prognostic implications. Int J Oncol 200221769–774. [DOI] [PubMed] [Google Scholar]
- 11.Rugge M, Ingravallo G, Farinati F.et al Cdx2 homeotic gene expression in gastric noninvasive neoplasia. Am J Surg Pathol 200428834–835. [PubMed] [Google Scholar]
- 12.Maruyama Y, Hanai H, Fujita M.et al Telomere length and telomerase activity in carcinogenesis of the stomach. Jpn J Clin Oncol 199727216–220. [DOI] [PubMed] [Google Scholar]
- 13.Tang R, Cheng A J, Wang J.et al Close correlation between telomerase expression and adenomatous polyp progression in multistep colorectal carcinogenesis. Cancer Res 1998584052–4054. [PubMed] [Google Scholar]
- 14.Yan P, Saraga E P, Bouzourene H.et al Telomerase activation in colorectal carcinogenesis. J Pathol 1999189207–212. [DOI] [PubMed] [Google Scholar]
- 15.Feng R H, Zhu Z G, Li J F.et al Inhibition of human telomerase in MKN‐45 cell line by antisense hTR expression vector induces cell apoptosis and growth arrest. World J Gastroenterol 20028436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krajewska M, Wang H G, Krajewski S.et al Immunohistochemical analysis of in vivo pattern of expression of CPP32 (Caspase‐3), a cell death protease. Cancer Res 1997571605–1613. [PubMed] [Google Scholar]
- 17.Shanmugathasan M, Jothy S. Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol Int 200050273–279. [DOI] [PubMed] [Google Scholar]
- 18.Zhivotovsky B, Samali A, Gahm A.et al The intracellular localization and translocation during apoptosis. Cell Death Differ 19996644–651. [DOI] [PubMed] [Google Scholar]
- 19.Ramuz O, Isnardon D, Devilard E. Constitutive nuclear localization and initial cytoplasmic apoptotic activation of endogenous caspase‐3 evidenced by confocal microscopy. Int J Exp Path 20038475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isobe N, Onodera H, Mori A.et al Caspase‐3 expression in human gastric carcinoma and its clinical significance. Oncology 200466201–209. [DOI] [PubMed] [Google Scholar]
- 21.Dixon M F, Genta R M, Yardley J H.et al Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996201161–1181. [DOI] [PubMed] [Google Scholar]
- 22.Genta R M. Recognizing atrophy: another step toward a classification of gastritis. Am J Surg Pathol 199620(Suppl 1)S23–S30. [DOI] [PubMed] [Google Scholar]
- 23.Rugge M, Genta R M. Staging gastritis: an international proposal. Gastroenterology 20051291807–1808. [DOI] [PubMed] [Google Scholar]
- 24.Rugge M, Genta R M. Staging and grading of chronic gastritis. Hum Pathol 200536228–233. [DOI] [PubMed] [Google Scholar]
- 25.Dowall J E, Willis P, Prescott R.et al Cell proliferation in type C gastritis affecting the intact stomach. J Clin Pathol 20005310784–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landis J R, Koch G G. The measurement of observer agreement for categorical data. Biometrics 197733159–174. [PubMed] [Google Scholar]
- 27.Correa P. The biological model of gastric carcinogenesis. IARC Sci Publ 2004157301–310. [PubMed] [Google Scholar]
- 28.Garcia S B, Park H S, Novelli M.et al Field cancerization, clonality, and epithelial stem cells: the spread of mutated clones in epithelial sheets. J Pathol 199918761–81. [DOI] [PubMed] [Google Scholar]
- 29.Mizoshita T, Inada K, Tsukamoto T.et al Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer 20014185–191. [DOI] [PubMed] [Google Scholar]
- 30.Brittan M, Wright N A. Stem cell in gastrointestinal structure and neoplastic development. Gut 200453899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loeffler M, Grossmann B. A stochastic branching model with formation of subunits applied to the growth of intestinal crypts. J Theor Biol 1991150175–191. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein W M, Goldstein N S. Gastric dysplasia and its management. Gastroenterology 19941071543–1545. [DOI] [PubMed] [Google Scholar]
- 33.Itskowitz S H, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology 20041261634–1648. [DOI] [PubMed] [Google Scholar]
- 34.Robert M E. Defining dysplasia in Barrett esophagus. J Clin Gastroenterol 200336(Suppl 5)S19–S25. [DOI] [PubMed] [Google Scholar]