Abstract
Background
A substantial proportion of patients with rheumatoid arthritis (RA) do not respond, or lose initial response, to adalimumab treatment. One explanation for non‐response is that patients develop anti‐adalimumab antibodies.
Objectives
To evaluate the incidence of formation of antibody against adalimumab and the association with serum adalimumab concentrations and clinical response.
Methods
In a cohort of 121 consecutive patients with RA treated with adalimumab, serum adalimumab concentrations and antibodies against adalimumab were measured together with clinical response variables before and up to 28 weeks after the start of treatment.
Results
Anti‐adalimumab antibodies were detected in 21 patients (17%) during 28 weeks of treatment. EULAR non‐responders had antibodies significantly more often than good responders (34% vs 5%; p = 0.032). Patients with antibodies showed less improvement in disease activity (mean (SD) delta DAS28 0.65 (1.35)) than patients without antibodies (mean delta DAS28 1.70 (1.35)) (p = 0.001). Patients with antibodies during follow‐up had lower serum adalimumab concentrations at 28 weeks than patients without antibodies (median 1.2 mg/l, range 0.0–5.6 vs median 11.0 mg/l, range 2.0–33.0, respectively; p<0.001). Good responders had higher serum adalimumab concentrations than moderate responders (p = 0.021) and non‐responders (p = 0.001). Concomitant methotrexate use was lower in the group with anti‐adalimumab antibodies (52%) than in the group without antibodies (84%) (p = 0.003).
Conclusions
Serum antibodies against adalimumab are associated with lower serum adalimumab concentrations and non‐response to adalimumab treatment.
Keywords: adalimumab, anti‐adalimumab antibodies, human anti‐human antibodies, rheumatoid arthritis
A substantial proportion of patients with rheumatoid arthritis (RA) still have persistent disease activity or flare of disease activity despite tumour necrosis factor (TNF)α blocking therapy.1 An explanation may be that antibodies are formed against these therapeutic agents. In patients with RA or Crohn's disease, human anti‐chimeric antibodies (HACAs) to infliximab have been reported. Initially, the clinical significance of these antibodies was uncertain. However, recent data on Crohn's disease indicate that these anti‐infliximab antibodies are associated with allergic reactions and a shorter duration of response.2 In RA, the development of antibodies against infliximab is associated with a reduced response to infliximab after treatment for an extended period of time.3 Simultaneous immunosuppressive therapy has been shown to reduce HACA formation.2,4
Adalimumab is a fully human antibody and therefore thought to be less immunogenic than chimeric antibodies.5 Nevertheless, it has previously been suggested that human anti‐human antibodies against adalimumab may develop as well, although the data are limited. Anti‐adalimumab antibodies were found in 12% of patients with RA receiving adalimumab monotherapy at a dose of 40 mg every other week.6,7 Contradictory results have been reported with regard to the influence of these antibodies on clinical response.6,7 We recently found high anti‐adalimumab concentrations in a patient with RA, which was associated with undetectable serum adalimumab concentrations and a diminished clinical response.8 This case report suggested that human anti‐human antibody formation may play an important role in some patients who do not respond to adalimumab treatment. This emphasises the need for further investigation with standardised analytical techniques into the effect of antibody formation on clinical response. Therefore, we evaluated adalimumab and anti‐adalimumab antibody concentrations in relation to clinical response in a cohort of patients with RA up to 28 weeks after initiation of treatment.
Patients and methods
Patients
This prospective observational cohort study consisted of 121 consecutive patients with RA treated with adalimumab at the Departments of Rheumatology of the Jan van Breemen Institute and the Academic Medical Center, Amsterdam. All patients fulfilled the American College of Rheumatology 1987 revised criteria for RA and had active disease, indicated by a disease activity score in 28 joints (DAS28) of ⩾3.2 despite earlier treatment with two disease‐modifying anti‐rheumatic drugs (DMARDs) including methotrexate at a dose of 25 mg a week or at the maximal tolerable dose, according to the Dutch consensus statement on the initiation and continuation of TNF blocking therapy in RA.9 Patients were treated with either adalimumab and concomitant DMARD or adalimumab alone. All patients used adalimumab 40 mg subcutaneously every other week. In patients with an inadequate response as judged by the treating rheumatologist, the dosing frequency of adalimumab could be increased to 40 mg a week. The study was approved by the medical ethics committee of the Slotervaart Hospital, BovenIJ Hospital, the Jan van Breemen Institute, and the Academic Medical Center/University of Amsterdam. All patients gave written informed consent.
Clinical response
Disease activity was assessed at baseline and after 4, 16 and 28 weeks of treatment using the DAS28 score.10 Clinical response was assessed by the European League Against Rheumatism (EULAR) criteria and the change in DAS28 score (delta DAS28).11 Serum samples were collected just before an injection with adalimumab at baseline, and after 4, 16 and 28 weeks.
Measurement of adalimumab concentrations
Trough serum adalimumab concentrations were measured by ELISA, based on the principle that adalimumab is captured through its ability to bind TNFα. Adalimumab was quantified as described previously for infliximab measurement with one modification.12 Adalimumab binding was assessed by incubation with biotinylated rabbit IgG directed to the adalimumab idiotype. The detection limit of the assay is about 0.001 mg/l.
Measurement of antibodies against adalimumab
Anti‐adalimumab antibodies were detected with a newly developed radioimmunoassay. The assay measures specific high‐avidity IgG antibodies against adalimumab by an antigen‐binding test as described previously for the detection of antibodies against infliximab.3 Serum (1 μl/test) was preincubated with Sepharose‐immobilised protein A (1 mg/test; Pharmacia, Uppsala, Sweden) in Freeze buffer (Sanquin, Amsterdam, the Netherlands). Non‐bound serum components were removed by washing before 50 μl 125I‐labelled F(ab)′2 fragment of adalimumab was added. After overnight incubation, non‐bound radiolabel was washed out and Sepharose‐bound radioactivity was measured. Test results were converted into arbitrary units per millilitre (AU/ml) by comparison with dilutions of a reference serum. The mean cut‐off value was set at 12 AU/ml (mean + 6 SD of the pretreatment values), which was derived from 100 healthy donors. In agreement with this, all sera of patients in this study contained less than 12 AU/ml anti‐adalimumab before they received their first adalimumab injection. Assay specificity was demonstrated by the absence of anti‐adalimumab in 25 sera containing high‐titre anti‐infliximab. Because the presence of adalimumab interferes with the assay, it is to be expected that in patients with high concentrations of adalimumab, anti‐adalimumab is underestimated or undetectable. The antibody test was considered positive when the antibody concentration exceeded 12 AU/ml and the adalimumab concentration was 5 mg/l or less. We occasionally observed patients with anti‐adalimumab concurrent with serum concentrations of adalimumab. Adalimumab and antibody concentrations were measured at baseline and 28 weeks after initiation of treatment. At week 4 and 16, antibody concentrations were determined only in serum of patients with an adalimumab concentration of less than 5 mg/l and in patients with anti‐adalimumab at week 28.
Statistical analysis
For differences between groups, we used the independent samples t test or Mann‐Whitney U test as appropriate. For differences between paired samples, we used the paired samples t test or Wilcoxon signed rank test. The threshold for significance was set at p<0.05. We used multiple logistic regression analysis to investigate the relationship between EULAR response and adalimumab concentration, as well as the presence of anti‐adalimumab antibodies and the influence of sex, age, methotrexate and prednisone use and dose on this relationship.
To analyse clinical response in patients with and without antibodies after 28 weeks of treatment, we used the last observation carried forward for patients who stopped treatment because of non‐response or adverse events. In a sub‐analysis, the effects of increasing the adalimumab dosing frequency to 40 mg a week were studied using the data obtained after the more frequent dosing.
Results
Patient characteristics
Most of the 121 patients who entered the study were female (79%), and the mean (SD) age was 53 (13) years (table 1). At baseline, patients had active disease, as indicated by a mean DAS28 score of 5.3 (1.1). Mean disease duration was 12 (10) years, and, on average, patients had failed 3.6 (1.6) prior DMARDs. Most patients used concomitant methotrexate (79%) with a mean dose of 19.4 (7.4) mg/week, and 34% used prednisone at a mean dose of 7.9 (4.3) mg/day. Fourteen (12%) patients used sulfasalazine and/or hydroxychloroquine in combination with methotrexate, two (2%) patients used concomitant hydroxychloroquine only, and 24 (20%) patients were receiving adalimumab monotherapy.
Table 1 Basic and clinical characteristics of patients at baseline.
| Total patient population (n = 121) | Patient with anti‐ adalimumab antibodies (n = 21) | Patients without anti‐adalimumab antibodies (n = 100) | |
|---|---|---|---|
| Age (years) | 53 (13) | 51 (16) | 54 (12) |
| Female | 95 (79%) | 17 (81%) | 78 (78%) |
| DMARD treatment | |||
| Prior DMARDs | 3.6 (1.6) | 3.4 (1.6) | 3.7 (1.6) |
| Prior biologicals | 34 (28%) | 10 (48%) | 24 (24%) |
| Methotrexate use | 95 (79%) | 11 (52%) | 84 (84%) |
| Methotrexate dose (mg/week) | 19.4 (7.4) | 17.0 (8.6) | 19.7 (7.3) |
| Methotrexate plus other DMARD use | 14 (12%) | 1 (5%) | 13 (13%) |
| No concomitant DMARD | 24 (20%) | 9 (43%) | 15 (15%) |
| Prednisone use | 41 (34%) | 9 (43%) | 32 (32%) |
| Prednisone dose (mg/day) | 7.9 (4.3) | 7.1 (2.3) | 8.1 (4.7) |
| Disease status | |||
| Disease duration (years) | 12 (10) | 11 (8) | 12 (11) |
| Rheumatoid factor positive | 93 (77%) | 16 (76%) | 77 (77%) |
| Erosive disease | 94 (78%) | 18 (86%) | 77 (77%) |
| Nodular disease | 29 (24%) | 5 (24%) | 24 (24%) |
| Erythrocyte sedimentation rate (mm/h) | 32 (25) | 42 (34) | 30 (23) |
| C reactive protein (mg/dl) | 24 (28) | 34 (38) | 22 (24) |
| DAS28 | 5.3 (1.1) | 5.5 (1.2) | 5.3 (1.1) |
DAS28, disease activity score in 28 joints; DMARD, disease‐modifying anti‐rheumatic drug.
Values are mean (SD) or number (%).
The percentage of patients with antibodies against adalimumab who used concomitant methotrexate was significantly lower than the percentage of patients without such antibodies (p = 0.003). The mean (SD) methotrexate dose did not differ significantly between antibody positive and negative patients.
Clinical response
After 28 weeks follow‐up, 109 of 121 patients were still receiving adalimumab treatment. Dosing frequency was increased in 10 patients. Of the remaining 99 patients, 77 (78%) were classified as responders according to the EULAR response criteria. The responders consisted of 34 (34%) good responders and 43 (43%) moderate responders. The mean improvement in DAS28 was 1.7 (1.3).
Twelve patients stopped treatment before week 28, six because of inefficacy (as concluded by the treating rheumatologist), and four because of side effects (chronic coughing, flu‐like symptoms, palpitations, dysuria, and fever of unknown origin). In one patient, adalimumab was stopped because of a suspected malignancy, and one patient died from an unknown cause after 16 weeks of treatment.
Antibodies against adalimumab
During 28 weeks of follow‐up, antibodies were detected in 21 (17%) patients. Nine (8%) patients were found to be positive for anti‐adalimumab at week 4 after the start of treatment, six (5%) patients at week 16, and another six (5%) at week 28. The serum concentrations of anti‐adalimumab showed two clusters, which could be separated at a cut‐off value of 100 AU/ml. Ten of the 21 patients had antibody concentrations that stayed below 100 AU/ml at all time points (range 13–57 AU/ml), and 11 patients had antibody concentrations above 100 AU/ml (range 115–10344 AU/ml). When antibody concentrations of 100 AU/ml or more were present, these remained high during treatment, and concentrations increased further over time. Antibody concentrations between 12 and 100 AU/ml were less stable over time and were intermittently detectable or undetectable.
The percentage of patients using concomitant methotrexate was significantly lower in patients with antibodies against adalimumab (52%) than in patients without such antibodies (84%) (p = 0.003) (table 1). The mean methotrexate dose did not differ significantly between anti‐adalimumab positive and negative patients. The mean methotrexate dose was 19.7 (7.3) mg/week in the patients without anti‐adalimumab antibodies, 21.9 (6.3) mg/week in the low‐antibody group (12–100 AU/ml), and 14.3 (8.9) mg/week in patients with high anti‐adalimumab antibody concentrations (>100 AU/ml). A trend towards a lower methotrexate dose was seen in the group with high antibody concentrations > 100 AU/ml (p = 0.056). Patients receiving concomitant methotrexate had a lower rate of antibody development than patients receiving adalimumab monotherapy (12% vs 38%).
Adalimumab concentrations
At 28 weeks, median trough adalimumab concentration was 10 mg/l and varied from undetectable to 28 mg/l in patients receiving adalimumab at a dose of 40 mg every other week. In patients who received 40 mg a week, the median trough adalimumab concentration was 12 mg/l and varied from 8 to 43 mg/l.
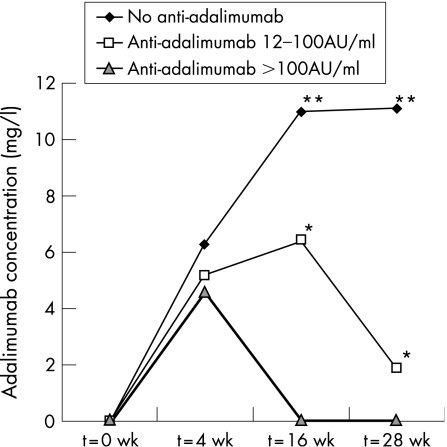
Figure 1 shows the course of the median trough adalimumab concentrations over 28 weeks of adalimumab. Serum adalimumab concentrations in patients with anti‐adalimumab antibodies were significantly lower than in patients without these antibodies (median 1.2 mg/l, range 0.0–5.6 vs median 11.0 mg/l, range 2.0–33.0; p<0.001).
Figure 1 Median serum trough adalimumab concentrations (mg/l) over time in patients with anti‐adalimumab antibody concentrations of 12–100 AU/ml and >100 AU/ml compared with patients without anti‐adalimumab antibodies. Adalimumab concentrations were significantly lower in patients with high anti‐adalimumab antibody concentrations than in those with low (*p<0.001) or absent (**p<0.001) antibodies at week 16. The difference in adalimumab concentrations between patients with low antibody concentrations and no antibodies was also significant (p = 0.001). At week 28, differences between all groups were highly significant (p<0.001).
Clinical response and anti‐adalimumab antibodies
EULAR non‐responders had anti‐adalimumab antibodies significantly more often than good responders (p = 0.006). After adjustment for sex and methotrexate dose, the presence of anti‐adalimumab antibodies remained a significant determinant of EULAR response (p = 0.032). EULAR non‐responders had anti‐adalimumab antibodies significantly more often than moderate responders (p = 0.039). However, this difference between non‐responders and moderate responders was no longer present after adjustment for methotrexate use (p = 0.148). The difference between good and moderate responders did not reach statistical significance.
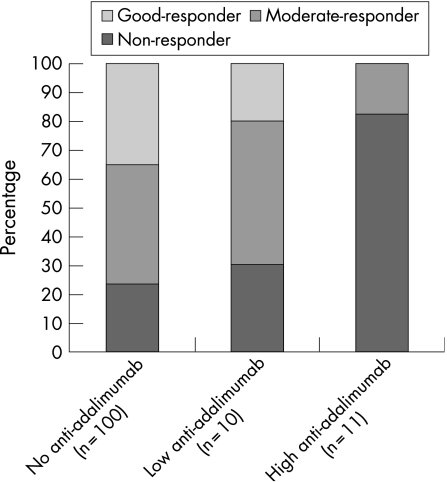
Of the 11 patients with anti‐adalimumab concentrations above 100 AU/ml, nine (82%) were non‐responders and two were moderate responders (18%). Of patients with anti‐adalimumab concentrations of 12–100 AU/ml (n = 10), only three were non‐responders, five were moderate responders, and two were good responders. Mean improvement in DAS28 in these patients was 0.20 (0.98). Of all patients in whom no antibodies against adalimumab were detected during the 28 weeks follow‐up, 23 (23%) were non‐responders, 41 (41%) moderate responsers, and 35 (35%) good responders. Figure 2 shows the relationship between EULAR response and presence or absence of anti‐adalimumab. Patients with anti‐adalimumab showed significantly less improvement in DAS28 (mean delta DAS28 0.65 (1.35)) than patients without anti‐adalimumab (mean delta DAS28 1.70 (1.35)) (p = 0.001). Figure 3 shows the relationship between DAS28 and adalimumab and anti‐adalimumab concentrations.
Figure 2 Relationship between EULAR response and presence or absence of anti‐adalimumab antibodies. Of the 11 patients with anti‐adalimumab antibody concentrations above 100 AU/ml, nine (82%) were non‐responders and two (18%) were moderate responders. Of the 10 patients with anti‐adalimumab concentrations of 12–100 AU/ml, only three were non‐responders (30%), five were moderate responders (50%), and two were good responders (20%). Of the 100 patients in whom no antibodies against adalimumab were detected during the 28 weeks follow‐up, 23 were non‐responders, 41 were moderate responders, and 35 were good responders. EULAR non‐responders had anti‐adalimumab antibodies significantly more often than good responders (p = 0.006) and moderate responders (p = 0.039), and most non‐responders were found in the group with high (>100 AU/ml) anti‐adalimumab concentrations compared with the group with low (12–100 AU/ml) anti‐adalimumab concentrations.
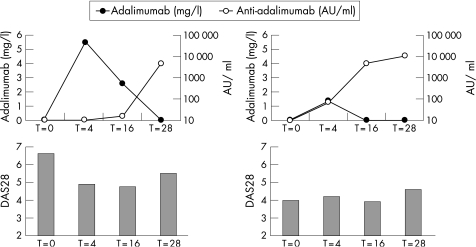
Figure 3 Serum adalimumab concentrations in relationship to anti‐adalimumab antibody concentrations and disease activity score in 28 joints (DAS28). The graphs represent two panels for two individual patients who had high anti‐adalimumab antibodies (>100 AU/ml) during 28 weeks follow‐up. The x‐axis represents time in weeks, and the left y‐axis represents adalimumab concentration in mg/l. The right y‐axis represents the anti‐adalimumab antibody concentration in AU/ml on a log scale. The DAS28 score is presented in bars.
Clinical response and adalimumab concentrations
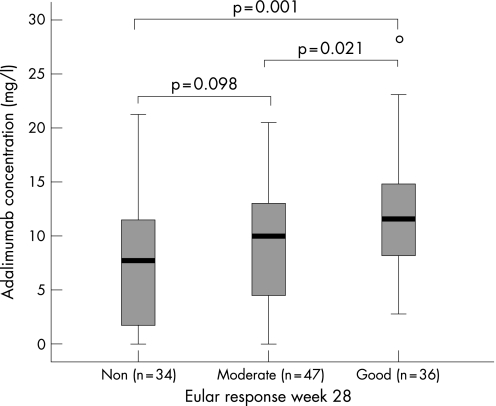
EULAR non‐responders had significantly lower serum adalimumab concentrations than good responders (median 5.4 mg/l, range 0.0–21.2 vs 9.8 mg/l, range 0.0–33.0; p = 0.001). Furthermore, non‐responders had lower serum adalimumab concentrations than moderate responders (p = 0.043). However, this difference was no longer significant after adjustment for methotrexate use (p = 0.098). Figure 4 shows the relationship between EULAR response and adalimumab concentration. Good responders had significantly higher serum adalimumab concentrations than moderate responders (p = 0.021).
Figure 4 Serum adalimumab concentrations (mg/l) in non‐responders, moderate responders, and good responders, according to the EULAR response criteria at week 28. EULAR non‐responders had significantly lower serum adalimumab concentrations than good responders (median 5.4 mg/l, range 0.0–21.2 vs 9.8 mg/l, range 0.0–33.0; p = 0.001). Non‐responders had lower serum adalimumab concentrations than moderate responders (p = 0.043). However, this difference was no longer significant after adjustment for methotrexate use (p = 0.098). Good responders had significantly higher serum adalimumab concentrations than moderate responders (p = 0.021). The boxplot presents the median and interquartile range, and the whiskers represent the 95th centiles.
Prednisone and methotrexate dose remained relatively stable in 17 of 21 patients who tested positive for anti‐adalimumab. In two of the 21 patients, low‐dose prednisone (5.0 and 7.5 mg/day) was stopped. The methotrexate dose (5 mg/week) was discontinued in one of the 21 patients, and in another patient methotrexate (15 mg/week) was started simultaneously with adalimumab.
Increased dosing frequency of adalimumab
In 10 patients (8%), the dosing frequency of adalimumab was increased to 40 mg a week, while concomitant methotrexate and prednisone dose remained stable. At the last visit before the dosing frequency was increased, seven patients were non‐responders, one was a moderate responder, and two were good responders (who subjectively perceived inefficacy). In five of the seven non‐responders, anti‐adalimumab antibodies were detectable, with antibody concentrations in the range 39–184 AU/ml. Anti‐adalimumab antibodies were no longer detectable after the increase in dosing frequency, and adalimumab concentrations significantly increased from a mean concentration of 2.0 (1.8) mg/l to 15.0 (8.0) mg/l (p = 0.043). Clinical response improved in the non‐responders, with a mean decrease in DAS28 of 1.7 (1.2). Figure 5 shows the effects of increasing the dosing frequency on DAS28 and adalimumab and anti‐adalimumab concentrations.
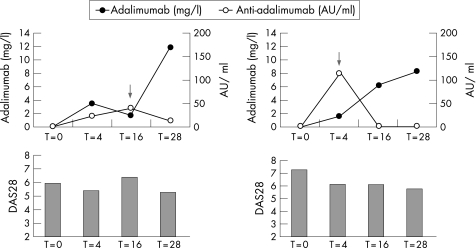
Figure 5 Changes in disease activity score in 28 joints (DAS28), adalimumab concentration and anti‐adalimumab antibody concentration after an increase in dosing frequency in two individual patients. In both non‐responders, the dosing frequency was increased to once a week, after which an increase in adalimumab concentration was observed (see arrow for time of dose increase). The x‐axis represents time in weeks, and the left y‐axis represents the adalimumab concentration in mg/l. The right y‐axis represents the anti‐adalimumab antibody concentration in AU/ml. The DAS28 score is presented in bars.
Discussion
After 28 weeks of treatment, anti‐adalimumab antibodies were found in 17% of the patients. Our results show that the presence of anti‐adalimumab is associated with diminished adalimumab concentrations and that especially high anti‐adalimumab concentrations are associated with the absence of detectable serum adalimumab. Furthermore, the presence of anti‐adalimumab as well as low serum adalimumab concentrations is associated with a diminished clinical response.
With infliximab treatment, HACAs were found in 29% of the patients during 30 weeks follow‐up.3 Infliximab is a chimeric monoclonal antibody, with a human constant region and murine variable regions. In adalimumab, a “fully human” antibody, the constant and variable regions are both “human”. Therefore, one can expect adalimumab to be less immunogenic. However, when comparing the relative immunogenicity, we have to take into account not only the extent of humanisation but also the differences in dosing schedules and route of administration of these two monoclonal antibodies.13 It is doubtful that any biological treatment will be completely without immunogenicity, but some may be more immunogenic than others.
Anti‐adalimumab antibodies have been previously reported in 12% of patients receiving adalimumab monotherapy 40 mg every other week for 26 weeks.7 In that study, no significant difference in ACR 20 response was found between patients with and without antibodies. In contrast, our data show that the presence of especially high concentrations of antibodies against adalimumab is associated with EULAR non‐response. Other data confirm our finding that a diminished clinical response is seen in patients with detectable antibodies compared with patients without antibodies.6 Differences between studies may be due to the use of different assays. Therefore, to come to an agreement on the immunological and clinical importance of antibody formation, it will be essential to further standardise the assays used for studying immunogenicity. Obviously, this is not only important for adalimumab but for all therapeutic monoclonal antibodies.
During 28 weeks follow‐up, high anti‐adalimumab antibody concentrations (>100 AU/ml) were associated with low serum trough adalimumab concentrations. The reduced adalimumab concentration in these patients is probably due to increased clearance of adalimumab via the formation of adalimumab–anti‐adalimumab immune complexes. This notion is supported by recent observations in patients with RA receiving 99 technetium‐labelled infliximab.14 In one HACA‐positive non‐responder, the formation of small immune complexes could be detected, which coincided with rapid clearance from the blood and high uptake of infliximab in the liver and spleen. Without adalimumab in the circulation, its function would logically be impaired. It is possible that a patient has adalimumab in the circulation in the interval between two dosings, and the concentration falls below the detection limit just before the next adalimumab injection. In this case, it would be possible for a patient to have a good response based on adequate adalimumab concentrations in the 2 week dosing interval despite the presence of antibodies against adalimumab and undetectable trough concentrations before the next injection. However, the fact that most patients with anti‐adalimumab concentrations above 100 AU/ml were non‐responders indicates that, overall, serum adalimumab concentrations were probably too low to have a therapeutic effect.
As there is competition for the assay in the presence of adalimumab, the incidence of anti‐adalimumab antibodies may be underestimated in all patients. Anti‐adalimumab antibodies may cause increased clearance and lower adalimumab concentrations in some patients while not yet detectable. This may be one explanation for the fact that serum adalimumab concentrations were significantly higher in EULAR good responders compared with moderate responders.
The question arises what causes anti‐adalimumab antibody formation in some patients, but not in others, and what could explain the difference between patients with high and low anti‐adalimumab concentrations. It is likely that immunosuppression with methotrexate reduces anti‐adalimumab formation, as shown previously for anti‐infliximab antibodies.4,6 Our data confirm the notion that concomitant methotrexate use reduces the rate of antibody development.
Anti‐adalimumab antibodies were no longer detectable in all five patients with anti‐adalimumab antibodies in whom the dosing frequency was increased. Increasing the dosing frequency might overload the capacity of the immune system to produce anti‐adalimumab, or alternatively induce immunotolerance. Interestingly, the disease activity (mean DAS28) improved considerably in these patients. It can be speculated that continuation of treatment with an increased dosing frequency of adalimumab can be effective in some patients with anti‐adalimumab antibody titres; however, dose escalation is not effective in all patients.8,15 In summary, these results show that anti‐adalimumab antibodies are associated with diminished adalimumab concentrations, which may be related to an increased clearance of serum adalimumab. Furthermore, a clear association was found between the presence of anti‐adalimumab antibodies and a diminished clinical response, indicating that the formation of anti‐adalimumab antibodies may be an explanation for non‐response to adalimumab treatment. Quantification of serum adalimumab and anti‐adalimumab antibody and learning more about the underlying factors that affect these concentrations should lead to more individualised and optimised treatment in the future.
Acknowledgements
We thank Els de Groot for preparation of the rabbit anti‐idiotype, and Henk de Vrieze and Shirley J. Janssen for performing the assays. We also acknowledge the research nurses, Marga Kammeijer‐Rippen and Margot P Colombijn, for performing clinical assessments.
Abbreviations
DAS28 - disease activity score in 28 joints
DMARD - disease‐modifying anti‐rheumatic drug
EULAR - European League Against Rheumatism
HACA - human anti‐chimeric antibody
RA - rheumatoid arthritis
TNF - tumour necrosis factor
Footnotes
Funding: Abbott Laboratories. CAW is supported by a grant (945‐02‐029) from the Netherlands Organization for Health Research and Development (ZonMw). The study sponsors had no involvement in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the paper for publication.
Competing interests: Professor Dijkmans and Professor Tak are members of the advisory board of Abbott and have received honoraria for lectures.
References
- 1.Olsen N J, Stein C M. New drugs for rheumatoid arthritis. N Engl J Med 20043502167–2179. [DOI] [PubMed] [Google Scholar]
- 2.Baert F, Noman M, Vermeire S, Van Assche G, D'Haens G, Carbonez A.et al Influence of immunogenicity on the long‐term efficacy of infliximab in Crohn's disease. N Engl J Med 2003348601–608. [DOI] [PubMed] [Google Scholar]
- 3.Wolbink G J, Vis M, Lems W F, Voskuyl A E, de Groot E, Nurmohamed M T.et al Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 200654711–715. [DOI] [PubMed] [Google Scholar]
- 4.Maini R N, Breedveld F C, Kalden J R, Smolen J S, Davis D, Macfarlane J D.et al Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor alpha monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998411552–1563. [DOI] [PubMed] [Google Scholar]
- 5.Hwang W Y, Foote J. Immunogenicity of engineered antibodies. Methods 2005363–10. [DOI] [PubMed] [Google Scholar]
- 6.FDA http: //fda.gov Adalimumab product approval information (accessed 1 Mar 2007)
- 7.van de Putte L B, Atkins C, Malaise M, Sany J, Russell A S, van Riel P L.et al Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis 200463508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartelds G M, Wolbink G J, Stapel S, Aarden L, Lems W F, Dijkmans B A C.et al High levels of human anti‐human antibodies to adalimumab in a patient not responding to adalimumab treatment. Ann Rheum Dis 2006651249–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furst D E, Breedveld F C, Kalden J R, Smolen J S, Burmester G R, Bijlsma J W.et al Updated consensus statement on biological agents, specifically tumour necrosis factor {alpha} (TNF{alpha}) blocking agents and interleukin‐1 receptor antagonist (IL‐1ra), for the treatment of rheumatic diseases, 2005. Ann Rheum Dis 200564(Suppl 4)iv2–NaN14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prevoo M L, van‘t Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 11.van Gestel A M, Anderson J J, van Riel P L, Boers M, Haagsma C J, Rich B.et al ACR and EULAR improvement criteria have comparable validity in rheumatoid arthritis trials. American College of Rheumatology European League of Associations for Rheumatology. J Rheumatol 199926705–711. [PubMed] [Google Scholar]
- 12.Wolbink G J, Voskuyl A E, Lems W F, de G E, Nurmohamed M T, Tak P P.et al Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 200564704–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobo E D, Hansen R J, Balthasar J P. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci 2004932645–2668. [DOI] [PubMed] [Google Scholar]
- 14.van der Laken C J, Voskuyl A E, Roos J C, Stigter van W M, de Groot E R, Wolbink G.et al Imaging and serum analysis of immune complex formation of radiolabeled infliximab and anti‐infliximab in responders and non‐responders to therapy for rheumatoid arthritis. Ann Rheum Dis 200766253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breedveld F C, Weisman M H, Kavanaugh A F, Cohen S B, Pavelka K, van Volenhoven R.et al The PREMIER study: a multicenter, randomized, double‐blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 20065426–37. [DOI] [PubMed] [Google Scholar]