Figure 1.
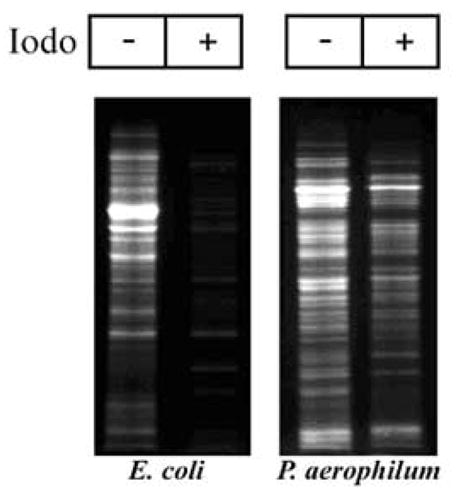
Abundance of disulfide-bonded proteins in P. aerophilum, as detected by fluorescent labeling. Whole cell lysate was reacted with iodoacetamide (+) to block free (thiol) cysteines. Following blocking, any disulfide bonds present were cleaved by reduction with TCEP and fluorescently labeled with CPM. When iodoacetamide is omitted (−) all cysteines are labeled. A comparison of corresponding lanes shows that a large fraction of P. aerophilum proteins contain disulfide bonds. E. coli cells serve as a control.
