Figure 2.
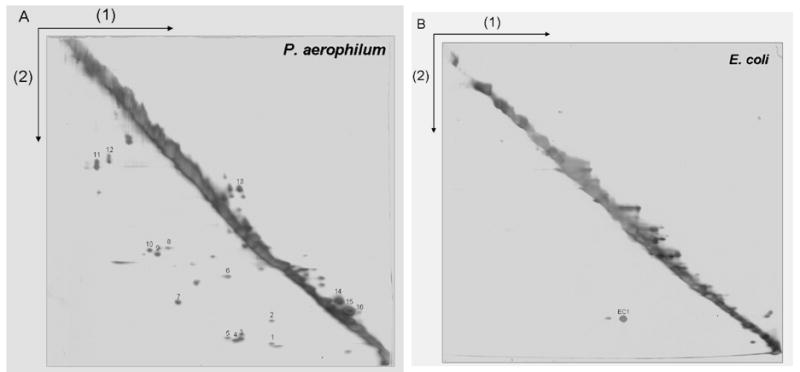
A 2-D diagonal gel electrophoresis method for identifying intermolecular disulfide bonded protein complexes. The first separation (1) is performed under non-reducing conditions so that disulfide bonds remain intact. Disulfide bonds are cleaved by reduction with DTT prior to the second electrophoretic separation (2). Proteins involved in intermolecular disulfide bonds appear as spots below the prominent diagonal, while spots above the diagonal mark certain intramolecularly disulfide-bonded proteins whose mobilities are retarded by reduction. Numerous disulfide bonded protein-protein complexes are visible in a cell lysate from (A) P. aerophilum, but not in (B) E. coli used as a control. P. aerophilum protein spots identified by mass spectrometry are numbered as in Table 2.
