Figure 5.
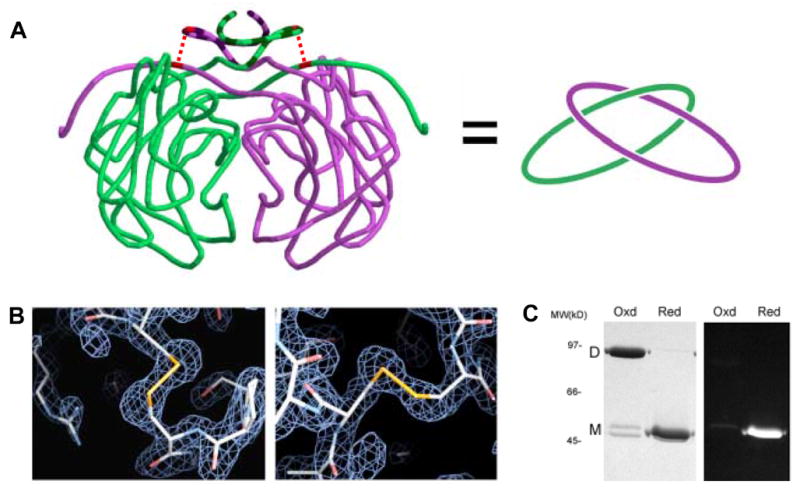
Intramolecular disulfide bonds leading to topological linkage or catenation of protein chains in P.aerophilum citrate synthase (PaCS). (A) Cartoon representation of interlinked PaCS chains. On the left, the protein backbone of the PaCS dimer is shown in a smoothed form to help clarify the chain topology. The region of the N-terminus (residues 1–24) that differs structurally in comparison to mesophilic homologs is indicated in darker striping. The topological connectivity is illustrated on the right. (B) Close up view of the disulfide bonds between Cys19 and Cys394 for both chains. Electron density maps calculated from diffraction data (based on phases from an omit-model) are shown in blue wireframe. (C) SDS-PAGE gel of purified recombinant PaCS. Under non-reducing (‘Oxd’) conditions, PaCS migrates at a molecular weight consistent with the dimeric form. Following treatment with the chemical reductant TCEP, the reduced (‘Red’) PaCS migrates as a monomer. A minor doublet in the oxidized lane likely corresponds to a mixture of linear and cyclized forms of the monomer. Fluorescent labelling with CPM indicates that no free thiols are present in the oxidized, dimeric form of PaCS.
