Abstract
Aim
To evaluate the effect of topically administered bevacizumab (Avastin) on experimental corneal neovascularisation in rats.
Methods
Silver nitrate sticks (75% silver nitrate, 25% potassium nitrate) were used to perform chemical cauterisation on the corneas of 16 eyes from 16 male Long Evans rats. For the following 7 days, the 10 eyes in the treatment group were instilled with bevacizumab 4 mg/ml drops twice daily, whereas the 6 eyes in the control group received placebo (normal saline drops twice daily). Digital photographs of the cornea were analysed to determine the area of cornea covered by neovascularisation as a percentage of the total corneal area.
Results
In the bevacizumab‐treated eyes, neovascularisation covered, on average, 38.2% (15.5%) (mean (SD)) of the corneal surface compared with 63.5% (5.0%) in the control group (p<0.02, Mann–Whitney U test).
Conclusion
Topically administered bevacizumab (Avastin) at a concentration of 4 mg/ml limits corneal neovascularisation following chemical injury in the male Long Evans rat model.
Corneal neovascularisation leads to scar formation, lipid deposition, immune rejection of corneal grafts and, therefore, significant visual impairment.1 It represents a major public health concern: worldwide, it is the common pathway to blindness from diseases such as trachoma and oncocerciasis, whereas in the US, 4% of the population has corneal neovascularisation.2,3
Vascular endothelial growth factor (VEGF) has been strongly implicated in corneal neovascularisation. Implanting a VEGF slow‐release polymer in the rabbit cornea stimulated corneal neovascularisation.4 Additionally, in experimental models of corneal neovascularisation, increased levels of corneal VEGF mRNA and protein levels, as well as increased levels of VEGF receptors, have been demonstrated.5,6,7,8 In humans, pathological studies have demonstrated that VEGF and its receptors are present in higher concentrations in corneal buttons with corneal neovascularisation than in normal corneas irrespective of the cause of neovascularisation.9,10
Conversely, VEGF inhibition has been shown to reduce corneal neovascularisation. For example, controlled‐release polyclonal anti‐VEGF antibody pellets implanted intrastromally inhibit experimental corneal neovascularisation.5 Additionally, a VEGF antagonist (the recombinant soluble form of the VEGF receptor Flt extracellular domain) or small interfering RNA against VEGF (or its receptors) inhibited herpes simplex‐induced corneal neovascularisation in mice.11,12,13
Bevacizumab (Avastin) is a full‐length humanised murine monoclonal antibody against the VEGF molecule (amino acid sequence is 93% of human origin and 7% of murine origin).14 It is commercially available and is approved by the US Food and Drug Administration (FDA) for use in the treatment of metastatic colorectal cancer, and phase III trials are underway for advanced breast and renal cancer.15,16 Anecdotal experience and case series have shown promising results for systemic or intravitreal use for exudative age‐related macular degeneration.17,18,19,20,21,22 Good results of intravitreal Avastin in the treatment of proliferative diabetic retinopathy showing regression of retina and iris neovascularisation,23,24 and macular oedema in central retinal vein occlusion have also been reported.25
The purpose of this study was to evaluate the effect of topical administration of Avastin in the prevention of experimentally induced corneal neovascularisation in a rat model.
Materials and methods
Sixteen male Long Evans pigmented rats weighing 200–250 g were used. Under general anaesthesia (induced by an intraperitoneally administered 94.7 mg/kg body weight ketamine hydrochloride and xylazine combination) supplemented by topical anaesthesia (0.5% proparacaine hydrochloride), the silver nitrate cauterisation technique described by Mahoney and Waterbury26 was used to induce corneal neovascularisation. One cornea of each animal was cauterised by pressing an applicator stick (with a diameter of 1.8 mm) coated with 75% silver nitrate/25% potassium nitrate (Arzol Chemical, Keen, New Hampshire, USA) to the central cornea for 10 s under the operating microscope. Excess silver nitrate was removed by rinsing the eyes with 5 ml of a balanced salt solution and then gently blotting the eyes with tissue paper. To increase the reproducibility of the injuries, a single investigator (PK) cauterised all animals.
Following cauterisation, the rats were randomised to one of two groups: group 1 (n = 10) received 4 mg/ml bevacizumab (Avastin) topically and group 2 (n = 6) received saline. Both were administered topically twice daily for 7 days. Treatment started immediately after cauterisation in the two groups. All animals were anaesthetised as described above, and their corneas were evaluated by slit‐lamp biomicroscopy on the third and sixth days. Corneal photographs were taken with ×25 magnification using a Nikon digital camera attached to the slit‐lamp microscope on the seventh day. Neovascularisation of each cornea was evaluated using the technique described by Mahoney and Waterbury26 by an examiner who was blinded to the treatment groups to minimise the observer bias. For each eye, the extent of burn stimulus response was scored as 0 (no blister, not raised above corneal surface), 1 (small blister, raised slightly above the surface), 2 (medium blister, raised moderately above the surface) or 3 (large blister). Only the corneas with a burn stimulus score of ⩾2 were included in the calculation of the mean burn stimulus and neovascularisation scores in each group. The corneal surface covered with neovascular vessels was measured on the photographs as the percentage of the total area of the cornea. Image analysis of each cornea was performed using an image processing and analysis software program (Image J V.1.31, Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, Maryland, USA). The area of neovascularisation was measured in terms of pixels and its ratio to the entire corneal area was determined as the percentage of corneal neovascularisation. A drawing of corneal blood vessels was made by one of the investigators to compare it with digital photos. This was carried out to ensure that no vascular area was missed during calculation. After scoring the burn stimulus and the percentage of neovascularisation for both groups, the animals were killed on the seventh day.
All the procedures involving animals were conducted in accordance with the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. All animals were housed in individual cages and maintained under standard conditions. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Tulane University Health Sciences Center, New Orleans, Louisiana, USA.
Tissue preparation/histopathology
Following sedation using the intraperitoneally administered ketamine hydrochloride and xylazine combination (94.7 mg/kg body weight), enucleation was performed after the animals were killed. Immediately after enucleation, the globes were penetrated with a 27 G needle, 1.0 mm from the limbus at the 3 and 9 o'clock meridians to allow the fixative to fill the eyes rapidly. The eyes were prepared for histological examination using 10% formaldehyde. After fixation for 24 h, they were removed from the fixative, and corneas were dehydrated and sectioned. The corneas were then soaked in xylene and paraffin wax, embedded in paraffin wax and cut at 8 μm for staining with H&E for light microscopy.
Sections were examined by dividing the corneas into two halves through the centre of the lesion and were evaluated with regard to the intensity of new vessels, polymorphonuclear leucocytes, oedema and fibroblastic activity. Light microscopic examination was performed on every section by an examiner who was blinded to the treatment groups.
Statistical analysis
The Mann–Whitney U test was used for comparisons. Significance was defined as a p value <0.05.
Results
The burn stimulus score was ⩾2 in all eyes. The mean burn stimulus scores were not statistically different between the treatment and the placebo groups (p>0.05, Mann–Whitney U test).
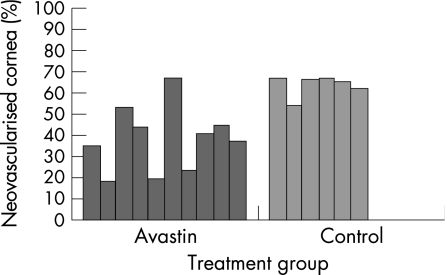
As fig 1 shows, in the bevacizumab‐treated eyes there was less corneal neovascularisation than in the control eyes after 7 days of cauterisation. In bevacizumab‐treated eyes corneal neovascularisation covered, on average, 38.2% (15.5%) (mean (SD)) of the corneal surface compared with 63.5% (5.0%) in the control group (p<0.02, Mann–Whitney U test). Therefore, bevacizumab decreased corneal neovascularisation by 40% (figs 2 and 3).
Figure 1 Normalised area of corneal neovascularisation in Avastin‐treated (n = 10) and control eyes (n = 6) after 7 days of corneal cauterisation. The difference is significant (p<0.02, Mann–Whitney U test).
Figure 2 Avastin‐treated eye after 7 days of corneal cauterisation.
Figure 3 Control eye after 7 days of corneal cauterisation.
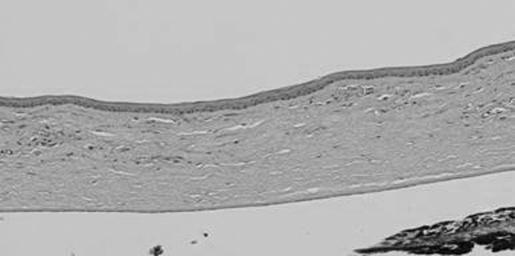
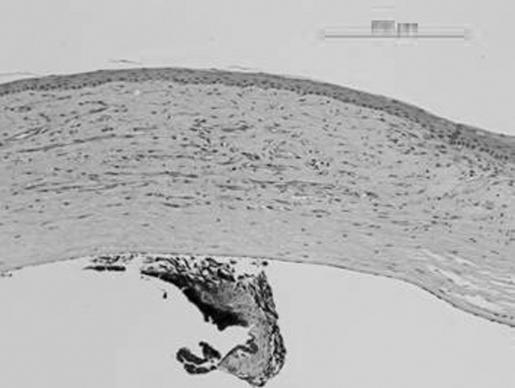
Light microscopy evaluation of the histological preparations was consistent with the slit‐lamp evaluation. The bevacizumab‐treated group had less neovascularisation and inflammation than the control eyes (figs 4 and 5). However, they still showed peripheral vascularisation of the cornea.
Figure 4 Histology (cornea with neovascularisation and moderate inflammation) of the control eye.
Figure 5 Histology (skip areas of vascularisation alternating with clear stroma) of the eye treated with Avastin.
Discussion
Although various compounds have been identified as inhibitors in experimental and clinical corneal neovascularization, including steroids,27,28,29 non‐steroidal anti‐inflammatory drugs,30,31,32 heparin,27,33 ciclosporin A,34 methotrexate35 and thalidomide,36 steroids have been the mainstay of treatment for corneal neovascularisation and corneal graft rejection in clinical practice. Steroids, however, are not always effective and chronic use may cause glaucoma, as well as precipitate infection or cataract formation.
The prominent role of VEGF in the pathophysiology of corneal neovascularisation has been demonstrated in experimental models of corneal neovascularisation,4,5,6,7,8 in experimental herpes simplex keratitis37 and in studies from human corneal buttons.9,10 Additionally, VEGF antagonism, whether at the protein or mRNA level, has been shown to reduce corneal neovascularisation and improve corneal graft survival in experimental animals.5,11,12,38,39
Our results suggest that Avastin (bevacizumab), a commercially available monoclonal anti‐VEGF antibody, also inhibits corneal neovascularisation in this rat model of corneal neovascularisation.
Although our results were highly significant (p<0.02, Mann–Whitney U test), inhibition of corneal neovascularisation was far from complete. There are several possible reasons for this. Firstly, it may be that twice daily administration is insufficient to effectively antagonise VEGF throughout the day: clearance of instilled bevacizumab through the tear outflow pathway may not allow binding of all available VEGF. Secondly, it is clear that cytokines other than VEGF (eg, transforming growth factor α and β1, and fibroblast growth factor) can induce corneal neovascularisation.3,9
Avastin has been used systemically for patients with advanced colorectal carcinoma and has been found to have a low incidence of significant adverse effects (induces mild to moderate hypertension and increases the rate of thrombosis in this patient population).40,41,42 It is unlikely that the miniscule doses delivered by topical administration would produce such adverse effects, although such safety data are, as yet, preliminary.43 Avastin has been injected intravitreally in humans, effectively controlling choroidal and retinal neovascularisation without inflammatory sequelae.18,19,20,22,23,24,25,44
We have shown that Avastin is efficacious in limiting corneal neovascularisation in an animal model. The next step is to use Avastin as an adjunct in a controlled clinical trial in the treatment of corneal neovascularisation and/or for high‐risk corneal graft recipients.
Abbreviations
VEGF - vascular endothelial growth factor
Footnotes
Competing interests: None.
References
- 1.Epstein R J, Stulting R D, Hendricks R L.et al Corneal neovascularization: pathogenesis and inhibition. Cornea 19876250–257. [DOI] [PubMed] [Google Scholar]
- 2.Lee P, Wang C C, Adamis A P. Ocular neovascularization: epidemiologic review. Surv Ophthalmol 199843245–269. [DOI] [PubMed] [Google Scholar]
- 3.Chang J H, Gabison E E, Kato T.et al Corneal neovascularization. Curr Opin Ophthalmol 200112242–249. [DOI] [PubMed] [Google Scholar]
- 4.Phillips G D, Stone A M, Jones B D.et al Vascular endothelial growth factor (rhVEGF165) stimulates direct angiogenesis in the rabbit cornea. In Vivo 19948961–965. [PubMed] [Google Scholar]
- 5.Amano S, Rohan R, Kuroki M.et al Requirement for vascular endothelial growth factor in wound‐ and inflammation‐related corneal neovascularization. Invest Ophthalmol Vis Sci 19983918–22. [PubMed] [Google Scholar]
- 6.Kvanta A, Sarman S, Fagerholm P.et al Expression of matrix metalloproteinase‐2 (MMP‐2) and vascular endothelial growth factor (VEGF) in inflammation‐associated corneal neovascularization. Exp Eye Res 200070419–428. [DOI] [PubMed] [Google Scholar]
- 7.Gan L, Fagerholm P, Palmblad J. Vascular endothelium growth factor (VEGF) and its receptor VEGFR‐2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand 200482557–563. [DOI] [PubMed] [Google Scholar]
- 8.Edelman J L, Castro M R, Wen Y. Correlation of VEGF expression by leukocytes with the growth and regression of blood vessels in the rat cornea. Invest Ophthalmol Vis Sci 1999401112–1123. [PubMed] [Google Scholar]
- 9.Cursiefen C, Rummelt C, Kuchle M. Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor α, and transforming growth factor β1 in human corneas with neovascularisation. Cornea 200019526–533. [DOI] [PubMed] [Google Scholar]
- 10.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci 2000412514–2522. [PubMed] [Google Scholar]
- 11.Kim B, Tang Q, Biswas P S.et al Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes. Am J Pathol 20041652177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng M, Schwarz M A, Lee S.et al Control of stromal keratitis by inhibition of neovascularisation. Am J Pathol 20011591021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binder P S. Herpes simplex keratitis. Surv Ophthalmol 197721313–331. [DOI] [PubMed] [Google Scholar]
- 14.Presta L G, Chen H, O'Connor S J.et al Humanization of an anti‐VEGF monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 1997574593–4599. [PubMed] [Google Scholar]
- 15.Miller K D, Chap L I, Holmes F A.et al Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 200523792–799. [DOI] [PubMed] [Google Scholar]
- 16.Yang J C, Haworth R M, Sherry R M.et al A randomized trial of bevacizumab, an anti‐VEGF antibody, for metastatic renal cancer. N Engl J Med 2003349427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michels S, Rosenfeld P J, Puliafito C A.et al Systemic bevacizumab (Avastin) therapy for neovascular age‐related macular degeneration: twelve week results of an uncontrolled open‐label clinical study. Ophthalmology 20051121035–1047. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld P J, Moshfeghi A A, Puliafito C A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age related macular degeneration. Ophthalm Surg Lasers Imaging 200536331–335. [PubMed] [Google Scholar]
- 19.Avery R L, Pieramici D J, Rabena M D.et al Intravitreal bevacizumab (Avastin) for neovascular age‐related macular degeneration. Ophthalmology 2006113363–372. [DOI] [PubMed] [Google Scholar]
- 20.Steinbrook R. The price of sight‐ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med 20063551409–1412. [DOI] [PubMed] [Google Scholar]
- 21.Moshfeghi A A, Rosenfeld P J, Puliafito C A.et al Systemic bevacizumab (Avastin) therapy for neovascular age‐related macular degeneration: twenty‐four‐week results of an uncontrolled open label clinical study. Ophthalmology 20061132002.e1–200312. [DOI] [PubMed] [Google Scholar]
- 22.Bashshur Z F, Bazarbachi A, Schakal A.et al Intravitreal bevacizumab for the management of choroidal neovascularization in age related macular degeneration. Am J Ophthalmol 20061421–9. [DOI] [PubMed] [Google Scholar]
- 23.Avery R L. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina 200626352–354. [DOI] [PubMed] [Google Scholar]
- 24.Spaide R F, Fisher Y L. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 200626275–278. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeld P J, Fung A E, Puliafito C A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging 200536336–339. [PubMed] [Google Scholar]
- 26.Mahoney J M, Waterbury L D. Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Curr Eye Res 19854531–535. [DOI] [PubMed] [Google Scholar]
- 27.Crum R, Szabo S, Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science 19852301375–1378. [DOI] [PubMed] [Google Scholar]
- 28.Lepri A, Benelli U, Bernardini N.et al Effect of low molecular weight heparan sulfate on angiogenesis in the rat cornea after chemical cauterization. J Ocul Pharm 199410273–280. [DOI] [PubMed] [Google Scholar]
- 29.Proia A D, Hirakata A, McInnes J S.et al The effect of angiostatic steroids and B‐cyclodextrin tetradecasulfate on corneal neovascularization in the rats. Exp Eye Res 199357693–698. [DOI] [PubMed] [Google Scholar]
- 30.Haynes W L, Proia A D, Klintworth G K. Effects of inhibitors of arachidonic acid metabolism on corneal neovascularization in the rat. Inv Ophthalmol Vis Sci 1989301588–1593. [PubMed] [Google Scholar]
- 31.Verbey N L, van Haeringen N J, de Jong P T. Modulation of immunogenickeratitis in rabbits by topical administration of inhibitors of lipoxygenase and cyclooxygenase. Curr Eye Res 19887361–368. [DOI] [PubMed] [Google Scholar]
- 32.Deutsch T A, Hughes W F. Suppressive effects of indomethacin on thermally induced neovascularization of rabbit corneas. Am J Ophthalmol 197987536–540. [DOI] [PubMed] [Google Scholar]
- 33.Benelli U, Bocci G, Danesi R.et al The heparan sulfate suleparoide inhibits rat corneal angiogenesis and in vitro neovascularization. Exp Eye Res 199867133–142. [DOI] [PubMed] [Google Scholar]
- 34.Lipman R M, Epstein R J, Hendricks R L. Suppression of corneal neovascularization with cyclosporine. Arch Ophthalmol 1992110405–407. [DOI] [PubMed] [Google Scholar]
- 35.Joussen A M, Kruse F E, Volcker H E.et al Topical application of methotrexate for inhibition of corneal angiogenesis. Graefes Arch Clin Exp Ophthalmol 1999237920–927. [DOI] [PubMed] [Google Scholar]
- 36.D'Amato R J, Loughnan M S, Flynn E.et al Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 1994914082–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng M, Deshpande S, Lee S.et al Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol 2001759828–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Binetruy‐Tournaire R, Demangel C, Malavaud B.et al Identification of a peptide blocking vascular endothelial growth factor (VEGF)‐mediated angiogenesis. EMBO J 2000191525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cursiefen C, Cao J, Chen L.et al Inhibition of lymphagiogenesis and hemangiogenesis after normal‐risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci 2004452666–2673. [DOI] [PubMed] [Google Scholar]
- 40.Kabbinavar F, Hurwitz H I, Fehrenbacher L.et al Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 20032160–65. [DOI] [PubMed] [Google Scholar]
- 41.Hurwitz H, Fehrenbacher L, Novotny W.et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 20043502335–2342. [DOI] [PubMed] [Google Scholar]
- 42.Fernando N H, Hurwitz H I. Targeted therapy of colorectal cancer: clinical experience with bevacizumab. Oncologist 20049(Suppl 1)11–18. [DOI] [PubMed] [Google Scholar]
- 43.Fung A E, Rosenfeld P J, Reichel E. The International Bevacizumab Safety Study: using the internet to assess drug safety worldwide. Br J Ophthalmol 2006901344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiss C, Michels S, Prager F.et al Evaluation of anterior chamber inflammatory activity in eyes treated with intravitreal bevacizumab. Retina 200626877–881. [DOI] [PubMed] [Google Scholar]