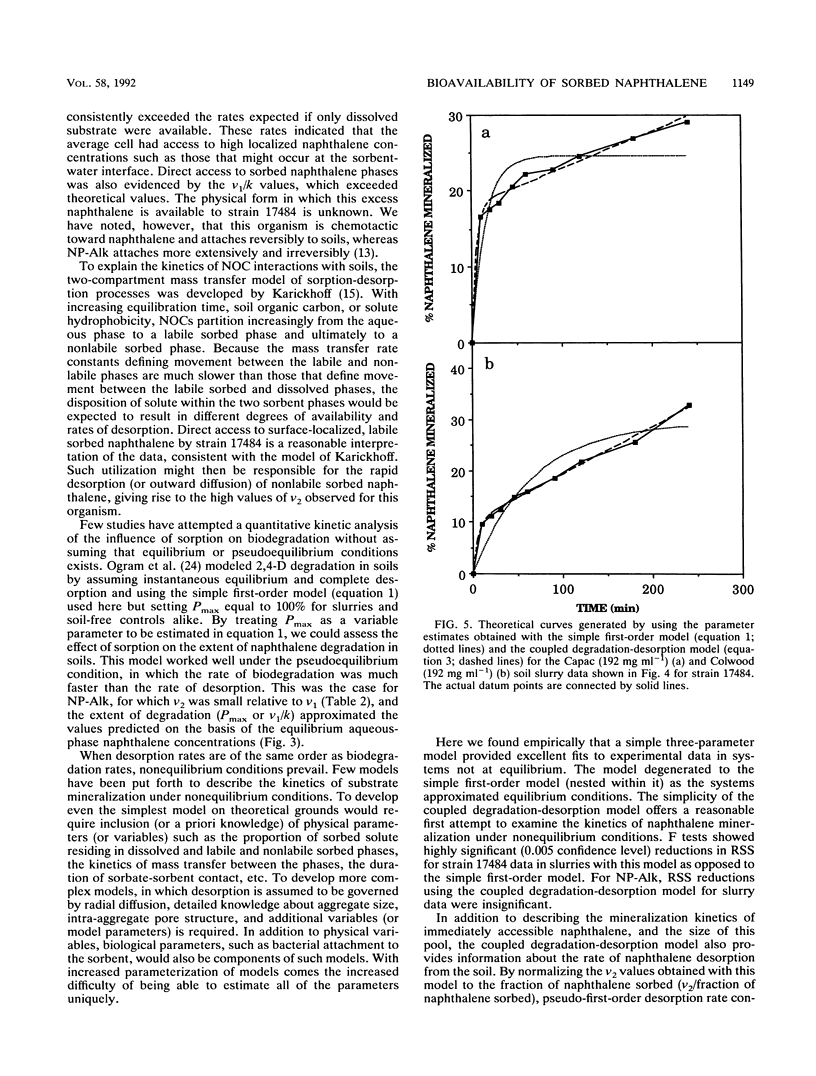
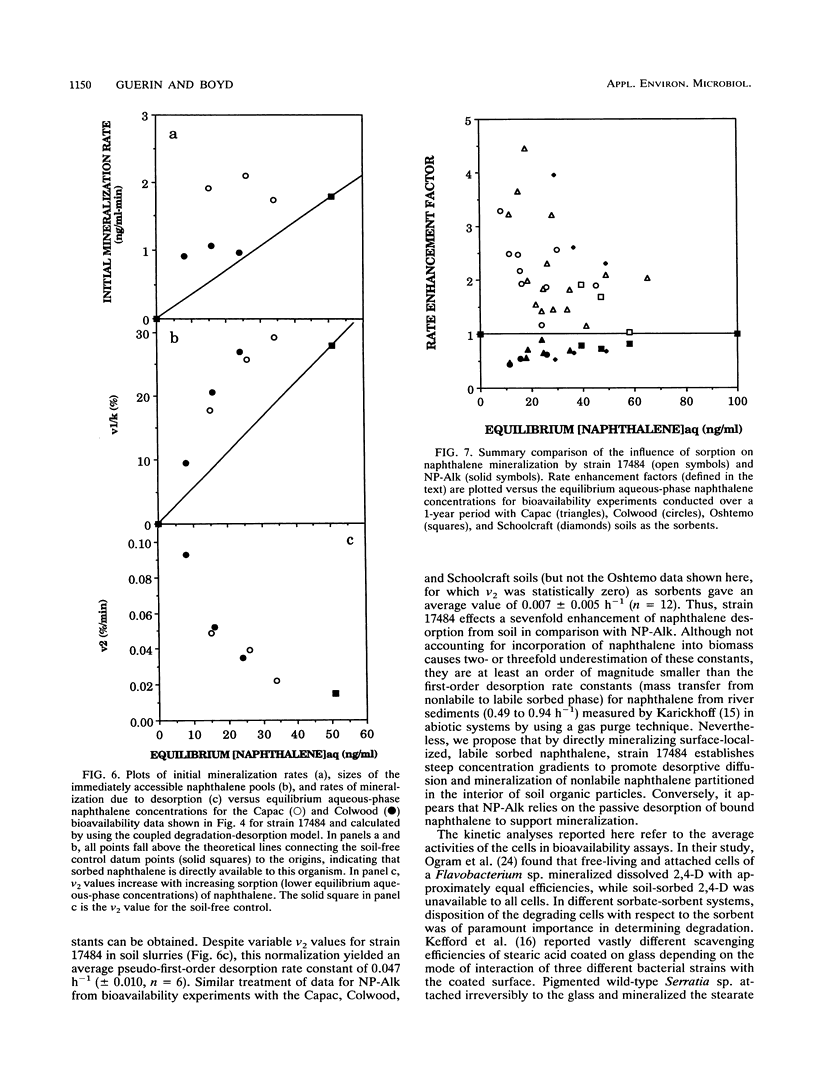
Abstract
Prediction of the fate of hydrophobic organic contaminants in soils is complicated by the competing processes of sorption and biodegradation. To test the hypothesis that sorbed naphthalene is unavailable to degradative microorganisms, we developed a simple kinetic method to examine the rates and extents of naphthalene degradation in soil-free and soil-containing systems in a comparison of two bacterial species. The method is predicated on the first-order dependence of the initial mineralization rate on the naphthalene concentration when the latter is below the Michaelis-Menten half-saturation constant (Km) for naphthalene for the organism under study. Rates and extents of mineralization were estimated by nonlinear regression analysis of data by using both a simple first-order model and a three-parameter, coupled degradation-desorption model described for the first time here. Bioavailability assays with two bacterial species (Pseudomonas putida ATCC 17484 and a gram-negative soil isolate, designated NP-Alk) gave dramatically different results. For NP-Alk, sorption limited both the rate and extent of naphthalene mineralization, in accordance with values predicted on the basis of the equilibrium aqueous-phase naphthalene concentrations. For strain 17484, both the rates and extents of naphthalene mineralization exceeded the predicted values and resulted in enhanced rates of naphthalene desorption from the soils. We conclude that there are important organism-specific properties which make generalizations regarding the bioavailability of sorbed substrates inappropriate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnsley E. A. Role and regulation of the ortho and meta pathways of catechol metabolism in pseudomonads metabolizing naphthalene and salicylate. J Bacteriol. 1976 Feb;125(2):404–408. doi: 10.1128/jb.125.2.404-408.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner W., Focht D. D. Deterministic three-half-order kinetic model for microbial degradation of added carbon substrates in soil. Appl Environ Microbiol. 1984 Jan;47(1):167–172. doi: 10.1128/aem.47.1.167-172.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou C. T., Peters L. J., Freed V. H. A physical concept of soil-water equilibria for nonionic organic compounds. Science. 1979 Nov 16;206(4420):831–832. doi: 10.1126/science.206.4420.831. [DOI] [PubMed] [Google Scholar]
- Connors M. A., Barnsley E. A. Naphthalene plasmids in pseudomonads. J Bacteriol. 1982 Mar;149(3):1096–1101. doi: 10.1128/jb.149.3.1096-1101.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith P. C., Fletcher M. Hydrolysis of Protein and Model Dipeptide Substrates by Attached and Nonattached Marine Pseudomonas sp. Strain NCIMB 2021. Appl Environ Microbiol. 1991 Aug;57(8):2186–2191. doi: 10.1128/aem.57.8.2186-2191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin W. F., Jones G. E. Two-stage mineralization of phenanthrene by estuarine enrichment cultures. Appl Environ Microbiol. 1988 Apr;54(4):929–936. doi: 10.1128/aem.54.4.929-936.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao R. C., Thomas R. S., Monkman J. L. Computerized gas chromatographic-mass spectrometric analysis of polycyclic aromatic hydrocarbons in environmental samples. J Chromatogr. 1975 Oct 29;112:681–700. doi: 10.1016/s0021-9673(00)99997-7. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J. Using linear and non-linear regression to fit biochemical data. Trends Biochem Sci. 1990 Dec;15(12):455–458. doi: 10.1016/0968-0004(90)90295-m. [DOI] [PubMed] [Google Scholar]
- Ogram A. V., Jessup R. E., Ou L. T., Rao P. S. Effects of sorption on biological degradation rates of (2,4-dichlorophenoxy) acetic acid in soils. Appl Environ Microbiol. 1985 Mar;49(3):582–587. doi: 10.1128/aem.49.3.582-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp R. J., Young R. L. Availability of organic chemicals for biodegradation in settled bottom sediments. Ecotoxicol Environ Saf. 1988 Feb;15(1):31–45. doi: 10.1016/0147-6513(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Simkins S., Alexander M. Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol. 1984 Jun;47(6):1299–1306. doi: 10.1128/aem.47.6.1299-1306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Zehnder A. J. Influence of interfaces on microbial activity. Microbiol Rev. 1990 Mar;54(1):75–87. doi: 10.1128/mr.54.1.75-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


