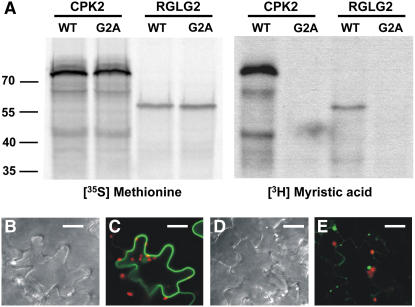
Figure 3.
Modification of RGLG2 by Myristic Acid.
(A) RGLG2 (WT) and the RGLG2 variant with Gly-2 changed to Ala (G2A) were incubated with either radioactive Met (left panels) or radioactive myristic acid (right panels) in an in vitro reaction. Wild-type RGLG2, but not the G2A mutant, can be modified by myristoylation. Gly-2 is the predicted linkage point to the fatty acid, and its absence does not allow modification. Protein kinase CPK2 served as a positive control.
(B) to (E) Mutant and wild-type RGLG2 proteins fused to GFP were observed in tobacco leaf epidermis cells at 2 d after agroinfection. Whereas the wild-type protein localizes to the cell periphery (C), the G2A mutant RGLG2-GFP protein does not accumulate well and forms aggregates (E), supporting a role for the N-terminal myristic acid as a membrane anchor. (B) and (D) show phase contrast images of (C) and (E), respectively. GFP signals are in green, and chlorophyll fluorescence of chloroplasts is visible in red. Bars = 10 μm.