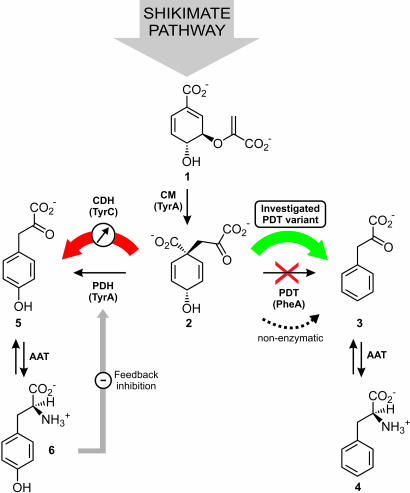
Fig. 1.
The engineered PDT selection system. The red X symbolizes the absence of the chorismate mutase–PDT (PheA) in the E. coli KA34 host strain. As a result of the pheA gene deletion, prephenate (2), generated from chorismate (1) by chorismate mutase (TyrA), is converted enzymatically to l-tyrosine (6) via 4-hydroxyphenylpyruvate (5) by prephenate dehydrogenase (TyrA) and AAT (aromatic amino acid aminotransferase). However, prephenate can accumulate because of feedback inhibition of TyrA by l-tyrosine. Spontaneous nonenzymatic conversion of prephenate to phenylpyruvate (3), which is subsequently transformed to l-phenylalanine (4) by AAT, leads to “leaky” growth under selective conditions. By introducing an externally regulable CDH (TyrC) from Z. mobilis, the intracellular prephenate concentration can be reduced, diminishing background growth in the absence of exogenously added phenylalanine and also affording tunable selection pressure on dehydratase surrogates.