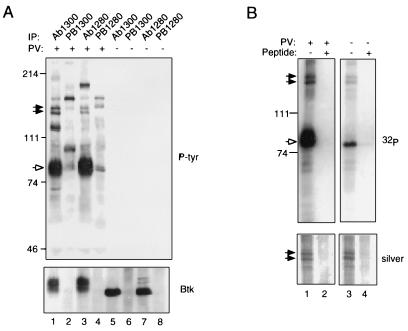
Figure 1.
Btk is associated with proteins of 135 and 140 kDa in B lymphoblastoid cells. (A) Coimmunoprecipitation assays. RAMOS cells were treated for 10 min with 1 mM pervanadate (lanes 1–4) or they were left untreated (lanes 5–8), and they were all lysed in the presence of 1% Nonidet P-40 (12). Immunoprecipitation was performed using 10 μl of preimmune sera (lanes 2, 4, 6, and 8) or anti-Btk antisera (lanes 1, 3, 5, and 7) as described (12). Phosphotyrosine-containing proteins present in immune complexes were detected by immunoblotting with anti-phosphotyrosine antibody 4G10 (Upper). Btk was detected using anti-Btk antibody Ab1280 (Lower). Btk is indicated by the open arrow; p135 and p140 by filled arrows. (B) Immune complex kinase assays. Btk was immunoprecipitated from lysates of pervanadate-stimulated (lanes 1 and 2) or unstimulated (lanes 3 and 4) RAMOS cells using affinity-purified antibody Ab1300 in the presence (lanes 2 and 4) or absence (lanes 1 and 2) of competitor peptide. Immune complex kinase assays were carried out as described (15). Protein was fractionated by 7.5% SDS-PAGE and detected by silver staining (Lower); after alkalai treatment to preferentially dephosphorylate phosphoserine and phosphothreonine residues, 32P was detected by autoradiography (Upper). Positions and masses of standards (in kDa) are indicated at left.