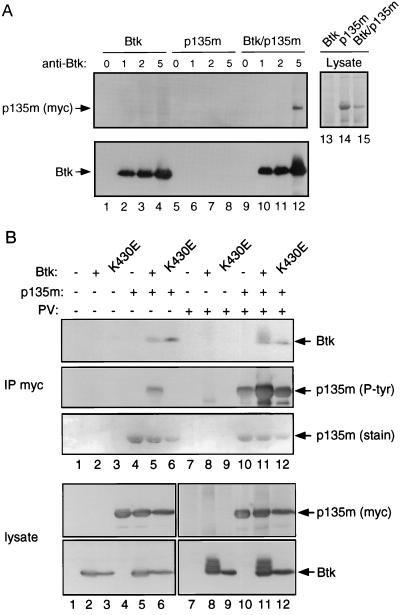
Figure 4.
Association of Btk with BAP-135 in transfected cells. (A) Cells expressing Btk (lanes 1–4), epitope-tagged BAP-135 (lanes 5–8), or both (lanes 9–12) were lysed in RIPA buffer containing protease inhibitors. Btk was immunoprecipitated in the presence of 0 μg (lanes 1, 5, and 9), 1 μg (lanes 2, 6, and 10), 2 μg (lanes 3, 7, and 11), or 5 μg (lanes 4, 8, and 12) of affinity-purified Ab1280. Precipitates were washed three times with RIPA buffer, once with a buffer containing 0.5 M NaCl and 50 mM Tris (pH 7.4) and twice with water. Proteins were separated by SDS/PAGE; epitope-tagged BAP-135 was detected by immunoblotting with antibody 9E10 (Upper, lanes 1–12) and Btk by Ab1280 (Lower, lanes 1–12). Total cell lysates were fractionated in lanes 13–15 at 1/10 the amount used for immunoprecipitation, and overexpressed BAP-135 was visualized by staining with amido black. (B) 293 cells expressing Btk (lanes 2 and 8), kinase-inactive Btk (lanes 3 and 9), epitope-tagged BAP-135 (lanes 4 and 10), Btk and epitope-tagged BAP-135 (lanes 5 and 11), kinase-inactive Btk and epitope-tagged BAP-135 (lanes 6 and 12), and cells transfected with vector alone (lanes 1 and 7) were lysed in the presence of 1% Nonidet P-40 (12). Before lysis, cells were treated with pervanadate (lanes 7–12) or untreated (lanes 1–6). Immunoprecipitation of epitope-tagged BAP-135 was carried out with antibody 9E10 and proteins were fractionated by SDS/PAGE (IP myc). In the upper three panels, Btk was detected by immunoblotting with Ab1280 (Btk, Top), tyrosine phosphorylated BAP-135 was detected by immunoblotting with antibody 4G10 (p135m [P-tyr], Middle) and BAP-135 protein was detected by amido black staining (p135m [stain], Bottom). In the lower two panels, total cell lysates were fractionated at one-tenth the amount used for immunoprecipitation (lysate); BAP-135 was detected by immunoblotting with 9E10 (p135m [myc], Upper) and Btk was detected with Ab1280 (Btk, Lower).