Abstract
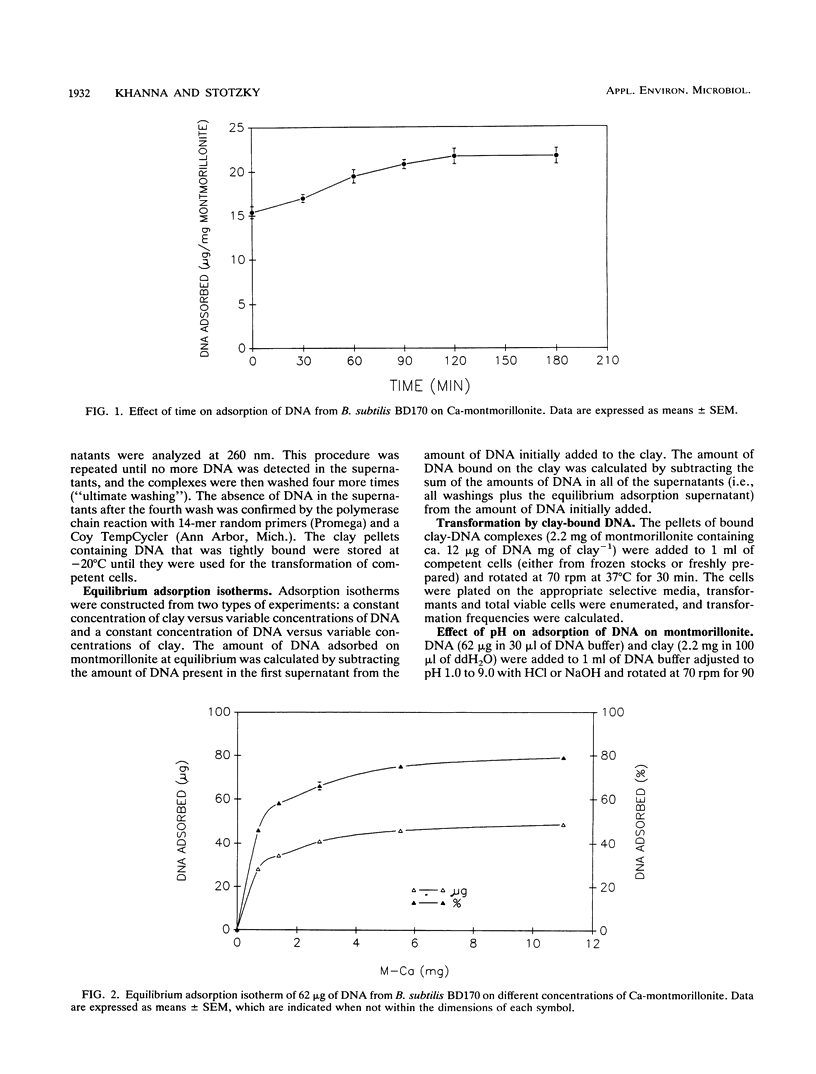
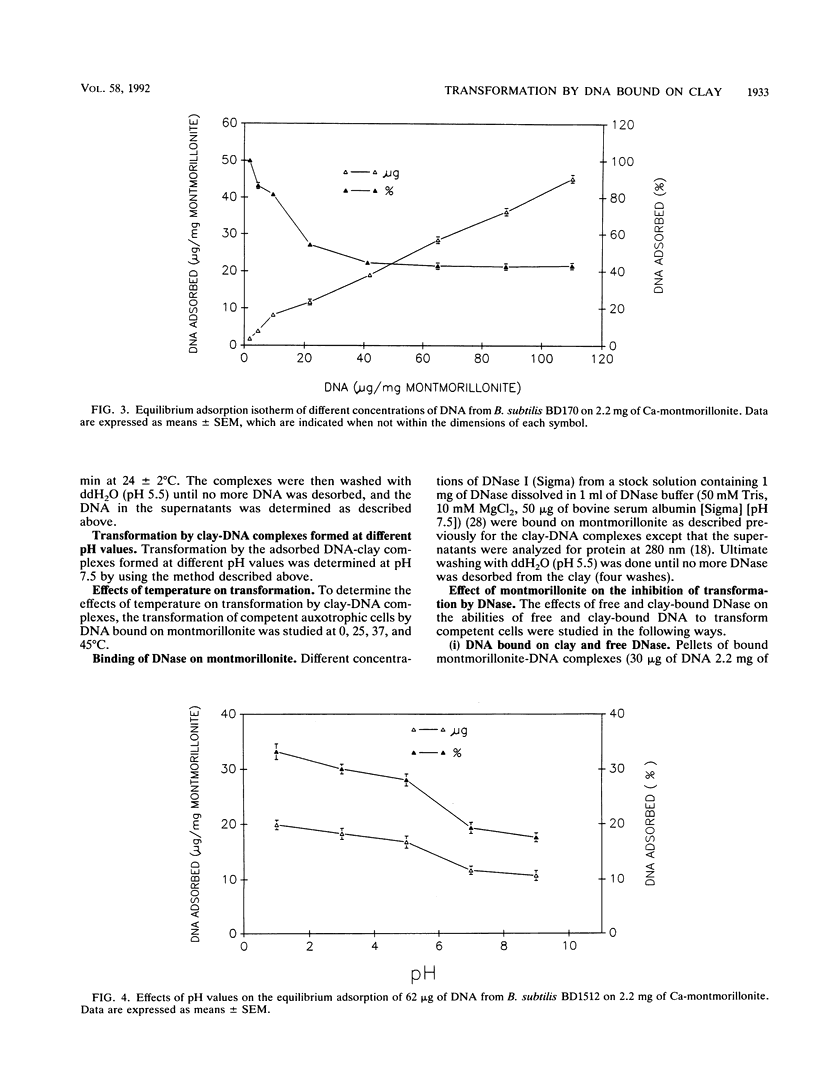
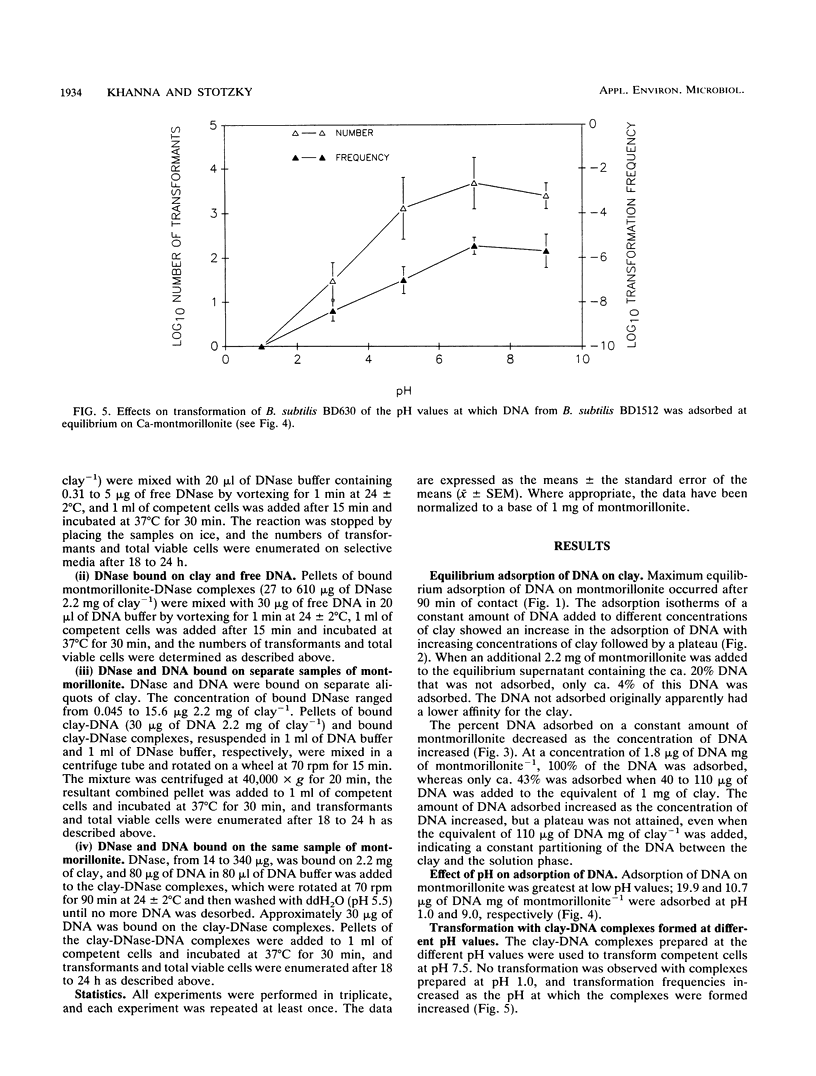
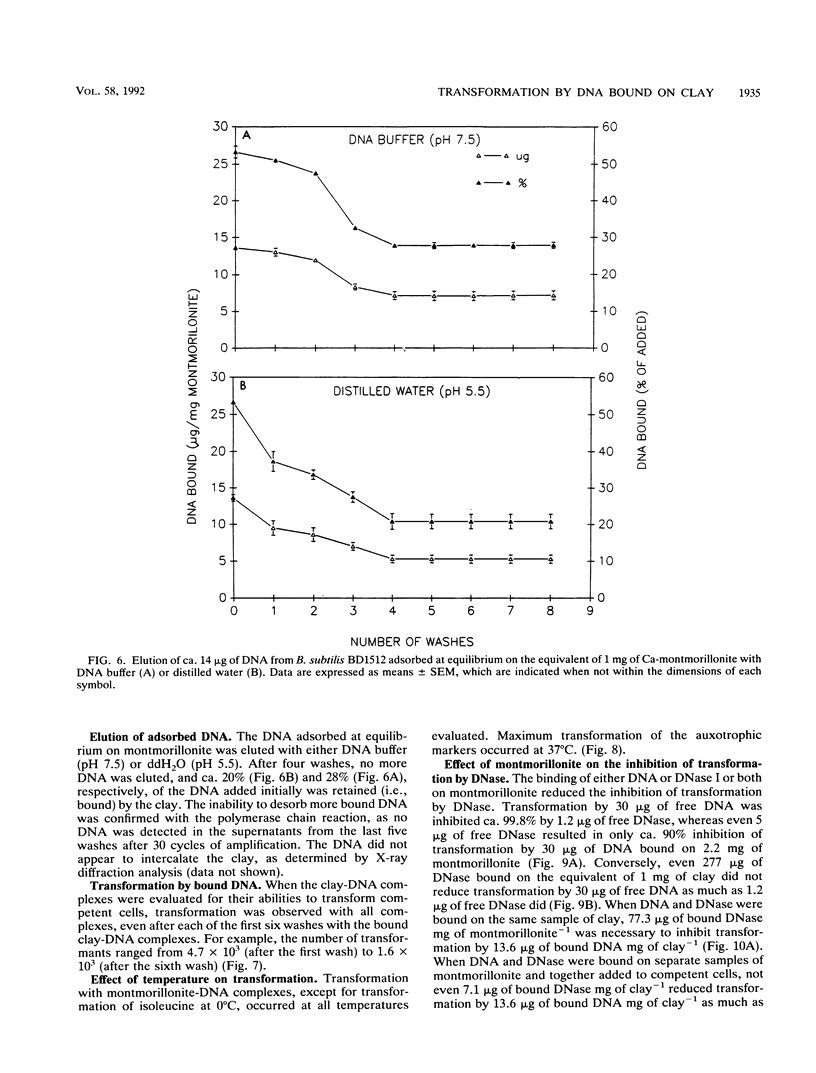
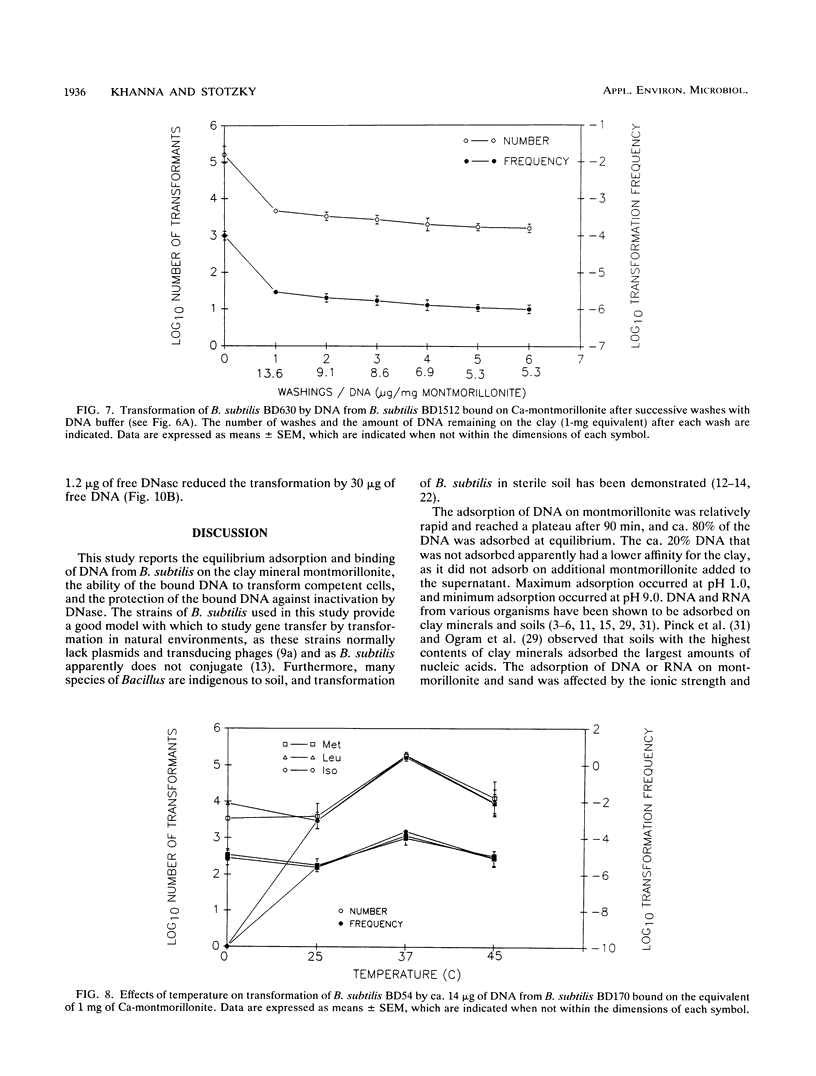
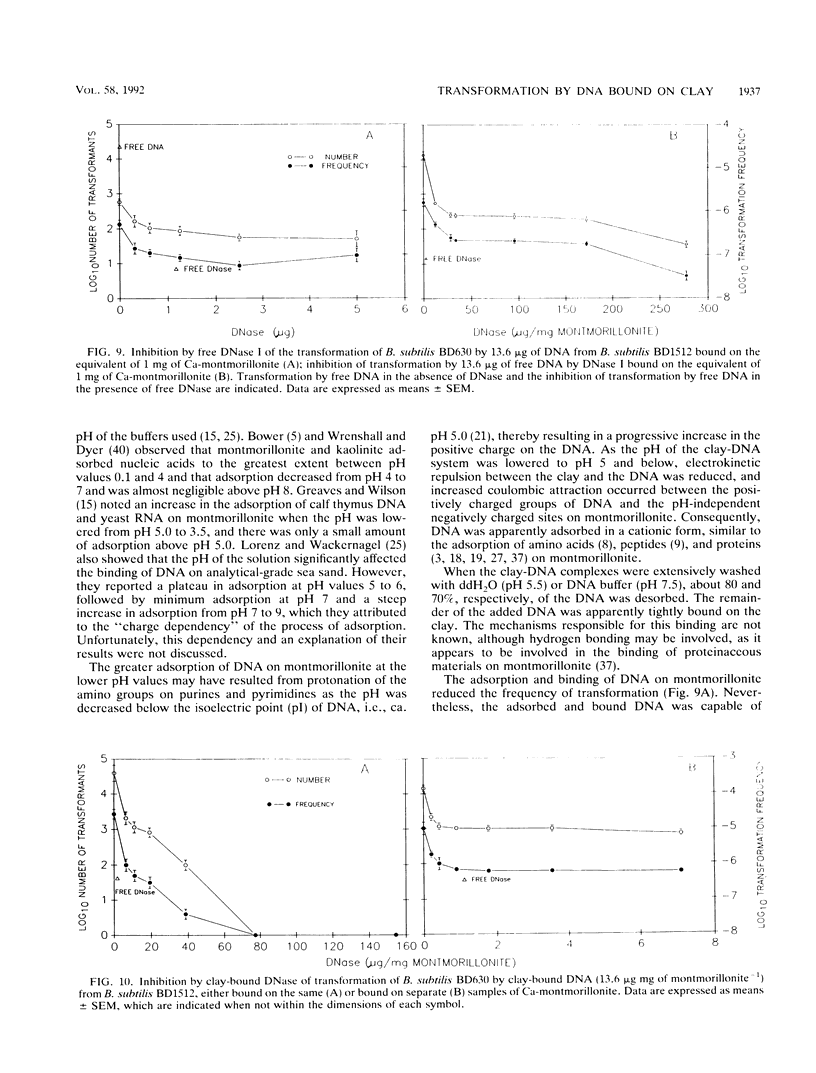
The equilibrium adsorption and binding of DNA from Bacillus subtilis on the clay mineral montmorillonite, the ability of bound DNA to transform competent cells, and the resistance of bound DNA to degradation by DNase I are reported. Maximum adsorption of DNA on the clay occurred after 90 min of contact and was followed by a plateau. Adsorption was pH dependent and was greatest at pH 1.0 (19.9 micrograms of DNA mg of clay-1) and least at pH 9.0 (10.7 micrograms of DNA mg of clay-1). The transformation frequency increased as the pH at which the clay-DNA complexes were prepared increased, and there was no transformation by clay-DNA complexes prepared at pH 1. After extensive washing with deionized distilled water (pH 5.5) or DNA buffer (pH 7.5), 21 and 28%, respectively, of the DNA remained bound. Bound DNA was capable of transforming competent cells (as was the desorbed DNA), indicating that adsorption, desorption, and binding did not alter the transforming ability of the DNA. Maximum transformation by bound DNA occurred at 37 degrees C (the other temperatures evaluated were 0, 25, and 45 degrees C). DNA bound on montmorillonite was protected against degradation by DNase, supporting the concept that "cryptic genes" may persist in the environment when bound on particulates. The concentration of DNase required to inhibit transformation by bound DNA was higher than that required to inhibit transformation by comparable amounts of free DNA, and considerably more bound than free DNase was required to inhibit transformation by the same amount of free DNA. Similarly, when DNA and DNase were bound on the same or separate samples of montmorillonite, the bound DNA was protected from the activity of DNase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aardema B. W., Lorenz M. G., Krumbein W. E. Protection of sediment-adsorbed transforming DNA against enzymatic inactivation. Appl Environ Microbiol. 1983 Aug;46(2):417–420. doi: 10.1128/aem.46.2.417-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton M. V., Barnett L. B. Adsorption of ribonucleic acid on Bentonite. Anal Biochem. 1969 Oct 15;32(1):150–154. doi: 10.1016/0003-2697(69)90115-8. [DOI] [PubMed] [Google Scholar]
- Coughter J. P., Stewart G. J. Genetic exchange in the environment. Antonie Van Leeuwenhoek. 1989;55(1):15–22. doi: 10.1007/BF02309615. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Graham J. B., Istock C. A. Genetic exchange in Bacillus subtilis in soil. Mol Gen Genet. 1978 Nov 9;166(3):287–290. doi: 10.1007/BF00267620. [DOI] [PubMed] [Google Scholar]
- Graham J. P., Istock C. A. Gene exchange and natural selection cause Bacillus subtilis to evolve in soil culture. Science. 1979 May 11;204(4393):637–639. doi: 10.1126/science.107592. [DOI] [PubMed] [Google Scholar]
- Lorenz M. G., Aardema B. W., Wackernagel W. Highly efficient genetic transformation of Bacillus subtilis attached to sand grains. J Gen Microbiol. 1988 Jan;134(1):107–112. doi: 10.1099/00221287-134-1-107. [DOI] [PubMed] [Google Scholar]
- Lorenz M. G., Wackernagel W. Adsorption of DNA to sand and variable degradation rates of adsorbed DNA. Appl Environ Microbiol. 1987 Dec;53(12):2948–2952. doi: 10.1128/aem.53.12.2948-2952.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGULIES M., VISHNIAC W. Dissimilation of glucose by the MX strain of Rhizopus. J Bacteriol. 1961 Jan;81:1–9. doi: 10.1128/jb.81.1.1-9.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Frischer M. E., Thurmond J. M. Gene transfer in marine water column and sediment microcosms by natural plasmid transformation. Appl Environ Microbiol. 1991 May;57(5):1509–1515. doi: 10.1128/aem.57.5.1509-1515.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Danner D. B., Deich R. A. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi: 10.1146/annurev.bi.50.070181.000353. [DOI] [PubMed] [Google Scholar]
- Stewart G. J., Carlson C. A. The biology of natural transformation. Annu Rev Microbiol. 1986;40:211–235. doi: 10.1146/annurev.mi.40.100186.001235. [DOI] [PubMed] [Google Scholar]
- Stewart G. J., Sinigalliano C. D. Detection of horizontal gene transfer by natural transformation in native and introduced species of bacteria in marine and synthetic sediments. Appl Environ Microbiol. 1990 Jun;56(6):1818–1824. doi: 10.1128/aem.56.6.1818-1824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotzky G., Babich H. Survival of, and genetic transfer by, genetically engineered bacteria in natural environments. Adv Appl Microbiol. 1986;31:93–138. doi: 10.1016/s0065-2164(08)70440-4. [DOI] [PubMed] [Google Scholar]


