Abstract
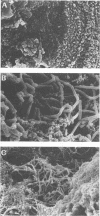
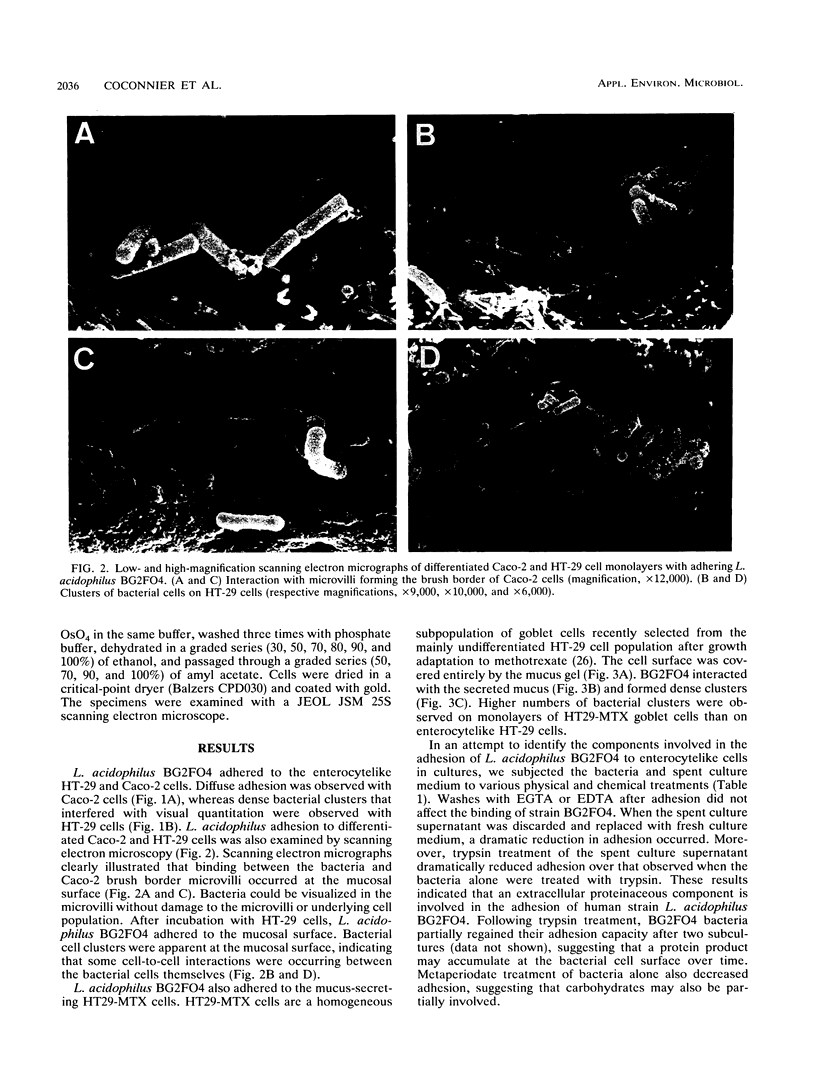
The adhesion of Lactobacillus acidophilus BG2FO4, a human stool isolate, to two human enterocytelike cell lines (Caco-2 and HT-29) and to the mucus secreted by a subpopulation of mucus-secreting HT29-MTX cells was investigated. Scanning electron microscopy revealed that the bacteria interacted with the well-defined apical microvilli of Caco-2 cells without cell damage and with the mucus secreted by the subpopulation of HT29-MTX cells. The adhesion to Caco-2 cells did not require calcium and involved an adhesion-promoting factor that was present in the spent supernatant of L. acidophilus cultures. This factor promoted adhesion of poorly adhering human Lactobacillus casei GG but did not promote adhesion of L. casei CNRZ 387, a strain of dairy origin. The adherence components on the bacterial cells and in the spent supernatant were partially characterized. Carbohydrates on the bacterial cell wall appeared to be partly responsible for the interaction between the bacteria and the extracellular adhesion-promoting factor. The adhesion-promoting factor was proteinaceous, since trypsin treatment dramatically decreased the adhesion of the L. acidophilus strain. The adhesion-promoting factor may be an important component of Lactobacillus species that colonize the gastrointestinal tract.
Full text
PDF

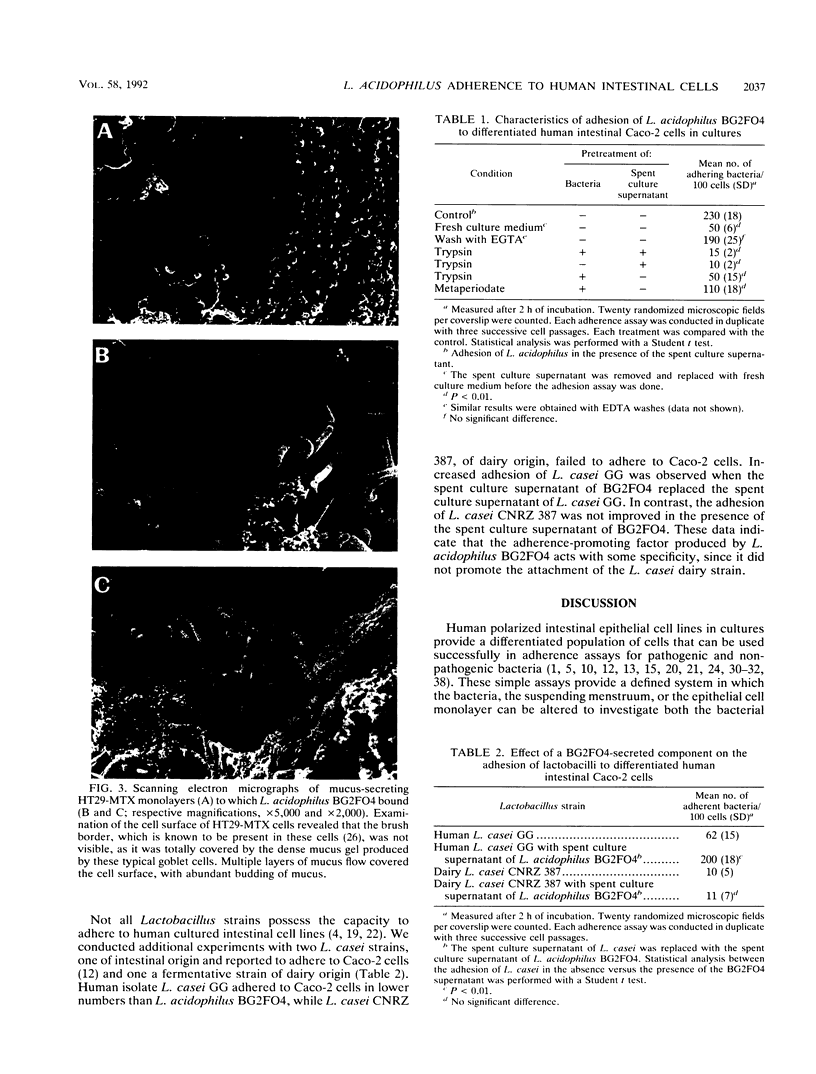


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler L., Mackay M. Reovirus 1 and 3 bind and internalise at the apical surface of intestinal epithelial cells. Virology. 1991 Sep;184(1):162–169. doi: 10.1016/0042-6822(91)90832-v. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Van Itallie C. M., Peterson M. D., Stevenson B. R., Carew E. A., Mooseker M. S. ZO-1 mRNA and protein expression during tight junction assembly in Caco-2 cells. J Cell Biol. 1989 Sep;109(3):1047–1056. doi: 10.1083/jcb.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker B. E., Fuller R. Adhesion of Lactobacilli to the chicken crop epithelium. J Ultrastruct Res. 1975 Jul;52(1):21–31. doi: 10.1016/s0022-5320(75)80019-0. [DOI] [PubMed] [Google Scholar]
- Conway P. L., Gorbach S. L., Goldin B. R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci. 1987 Jan;70(1):1–12. doi: 10.3168/jds.S0022-0302(87)79974-3. [DOI] [PubMed] [Google Scholar]
- Conway P. L., Kjelleberg S. Protein-mediated adhesion of Lactobacillus fermentum strain 737 to mouse stomach squamous epithelium. J Gen Microbiol. 1989 May;135(5):1175–1186. doi: 10.1099/00221287-135-5-1175. [DOI] [PubMed] [Google Scholar]
- Darfeuille-Michaud A., Aubel D., Chauviere G., Rich C., Bourges M., Servin A., Joly B. Adhesion of enterotoxigenic Escherichia coli to the human colon carcinoma cell line Caco-2 in culture. Infect Immun. 1990 Apr;58(4):893–902. doi: 10.1128/iai.58.4.893-902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990 Nov;162(5):1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P. Biogenesis and intracellular transport of intestinal brush border membrane hydrolases. Use of antibody probes and tissue culture. Subcell Biochem. 1988;12:155–219. doi: 10.1007/978-1-4899-1681-5_5. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985 Sep;101(3):838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson A., Szewzyk R., Conway P. L. Characteristics of the adhesive determinants of Lactobacillus fermentum 104. Appl Environ Microbiol. 1991 Feb;57(2):499–502. doi: 10.1128/aem.57.2.499-502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerneis S., Bilge S. S., Fourel V., Chauviere G., Coconnier M. H., Servin A. L. Use of purified F1845 fimbrial adhesin to study localization and expression of receptors for diffusely adhering Escherichia coli during enterocytic differentiation of human colon carcinoma cell lines HT-29 and Caco-2 in culture. Infect Immun. 1991 Nov;59(11):4013–4018. doi: 10.1128/iai.59.11.4013-4018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeman E. G., Klaenhammer T. R. Adherence of Lactobacillus species to human fetal intestinal cells. J Dairy Sci. 1982 Nov;65(11):2063–2069. doi: 10.3168/jds.S0022-0302(82)82462-4. [DOI] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuffleur T., Barbat A., Dussaulx E., Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990 Oct 1;50(19):6334–6343. [PubMed] [Google Scholar]
- Luchansky J. B., Tennant M. C., Klaenhammer T. R. Molecular cloning and deoxyribonucleic acid polymorphisms in Lactobacillus acidophilus and Lactobacillus gasseri. J Dairy Sci. 1991 Oct;74(10):3293–3302. doi: 10.3168/jds.S0022-0302(91)78515-9. [DOI] [PubMed] [Google Scholar]
- McCarthy D. M., Lin J. H., Rinckel L. A., Savage D. C. Genetic transformation in Lactobacillus sp. strain 100-33 of the capacity to colonize the nonsecreting gastric epithelium in mice. Appl Environ Microbiol. 1988 Feb;54(2):416–422. doi: 10.1128/aem.54.2.416-422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeser J. R., Chambaz A., Golliard M., Link-Amster H., Fryder V., Kolodziejczyk E. Adhesion of colonization factor antigen II-positive enterotoxigenic Escherichia coli strains to human enterocytelike differentiated HT-29 cells: a basis for host-pathogen interactions in the gut. Infect Immun. 1989 Dec;57(12):3727–3734. doi: 10.1128/iai.57.12.3727-3734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi P., Tall B. D., Russell R. G., Detolla L. J., Morris J. G., Jr Development of an in vitro model for study of non-O1 Vibrio cholerae virulence using Caco-2 cells. Infect Immun. 1990 Oct;58(10):3415–3424. doi: 10.1128/iai.58.10.3415-3424.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G., Bruce A. W., McGroarty J. A., Cheng K. J., Costerton J. W. Is there a role for lactobacilli in prevention of urogenital and intestinal infections? Clin Microbiol Rev. 1990 Oct;3(4):335–344. doi: 10.1128/cmr.3.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Svensson L., Finlay B. B., Bass D., von Bonsdorff C. H., Greenberg H. B. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J Virol. 1991 Aug;65(8):4190–4197. doi: 10.1128/jvi.65.8.4190-4197.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadström T., Andersson K., Sydow M., Axelsson L., Lindgren S., Gullmar B. Surface properties of lactobacilli isolated from the small intestine of pigs. J Appl Bacteriol. 1987 Jun;62(6):513–520. doi: 10.1111/j.1365-2672.1987.tb02683.x. [DOI] [PubMed] [Google Scholar]
- Zweibaum A., Pinto M., Chevalier G., Dussaulx E., Triadou N., Lacroix B., Haffen K., Brun J. L., Rousset M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985 Jan;122(1):21–29. doi: 10.1002/jcp.1041220105. [DOI] [PubMed] [Google Scholar]