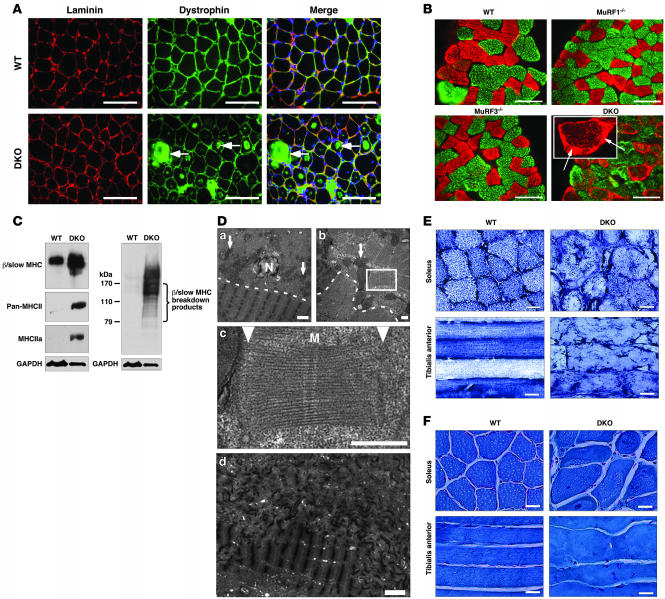
Figure 2. DKO mice display MSM.
(A) Immunohistochemistry of soleus muscle using anti-laminin and anti-dystrophin antibodies. Dystrophin immunostaining was observed within myofibers only in DKO muscle. (B) Immunohistochemistry of soleus muscle using anti–β/slow MHC (red) and anti–pan-MHCII (green) antibodies. Inset shows a representative myofiber with subsarcolemmal β/slow MHC accumulation (arrows). (C) Immunoblotting of proteins from the soluble (left blot) and particulate (right blot) fractions of the soleus revealed an accumulation of β/slow MHC in DKO muscle. Anti-GAPDH was used as a control. (D) Electron microscopy of longitudinal (top left and bottom) and cross (top right and middle) sections of soleus muscle displayed accumulation of granular material and unassociated myosins in DKO myofibers. Arrows indicate mitochondria. Dotted lines indicate separation of inner myofiber core (below the line) from accumulating myosin (above the line). Middle panel shows an enlargement of the area within the box at top right. Arrowheads point toward abnormal Z-lines. Bottom: Z-line streaming found in skeletal muscle myopathies is seen in the top of this panel. M, M-line; N, nucleus. (E) NADH stain of soleus and tibialis anterior muscles shows disorganized mitochondria in DKO muscle. (F) Modified Gomori trichrome stain of soleus and tibialis anterior muscles shows no ragged red fiber myopathy. Scale bars: 100 μm (A and B); 2 μm (D, top left and bottom); 0.5 μm (D, top right and middle); 20 μm (E and F).