Abstract
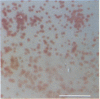
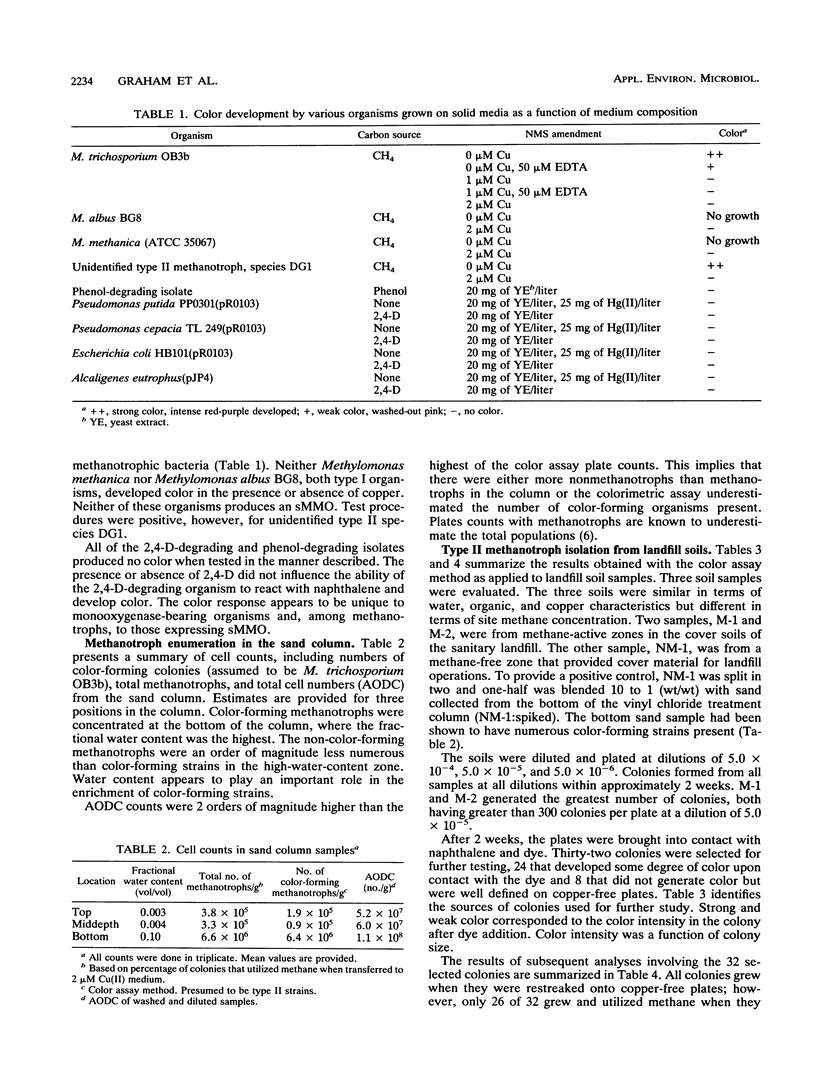
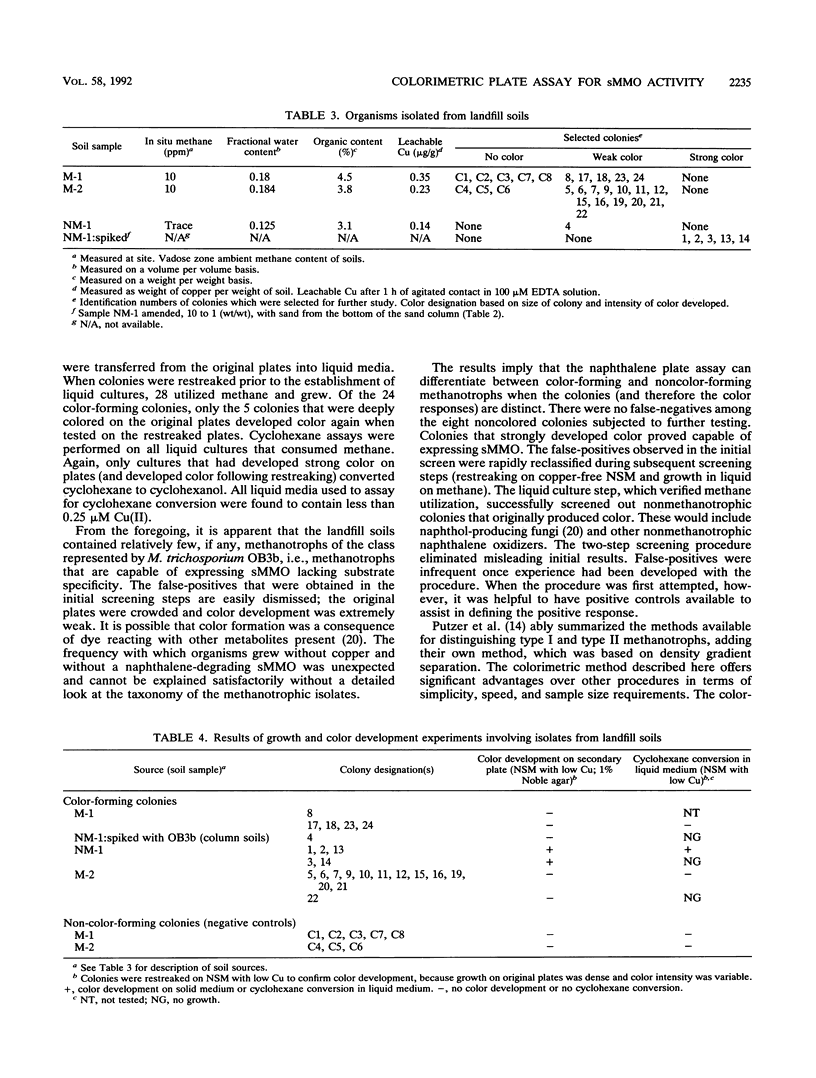
A straightforward method is described for screening methanotrophic colonies for soluble methane monooxygenase (sMMO) activity on solid media. Such activity results in the development of a colored complex between 1-naphthol, which is formed when sMMO reacts with naphthalene, and o-dianisidine (tetrazotized). Methanotrophic colonies expressing sMMO turned deep purple when exposed successively to naphthalene and o-dianisidine. The method was evaluated within the contexts of two potential applications. The first was for the enumeration of Methylosinus trichosporium OB3b in a methane-amended, unsaturated soil column dedicated to vinyl chloride treatment. The second application was for the isolation and enumeration of sMMO-bearing methanotrophs from sanitary landfill soils. The technique was effective in both applications.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brusseau G. A., Tsien H. C., Hanson R. S., Wackett L. P. Optimization of trichloroethylene oxidation by methanotrophs and the use of a colorimetric assay to detect soluble methane monooxygenase activity. Biodegradation. 1990;1(1):19–29. doi: 10.1007/BF00117048. [DOI] [PubMed] [Google Scholar]
- Fox B. G., Borneman J. G., Wackett L. P., Lipscomb J. D. Haloalkene oxidation by the soluble methane monooxygenase from Methylosinus trichosporium OB3b: mechanistic and environmental implications. Biochemistry. 1990 Jul 10;29(27):6419–6427. doi: 10.1021/bi00479a013. [DOI] [PubMed] [Google Scholar]
- Harker A. R., Olsen R. H., Seidler R. J. Phenoxyacetic acid degradation by the 2,4-dichlorophenoxyacetic acid (TFD) pathway of plasmid pJP4: mapping and characterization of the TFD regulatory gene, tfdR. J Bacteriol. 1989 Jan;171(1):314–320. doi: 10.1128/jb.171.1.314-320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. D., Palumbo A. V., Herbes S. E., Lidstrom M. E., Tyndall R. L., Gilmer P. J. Trichloroethylene biodegradation by a methane-oxidizing bacterium. Appl Environ Microbiol. 1988 Apr;54(4):951–956. doi: 10.1128/aem.54.4.951-956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhuis R., Vink R. L., Janssen D. B., Witholt B. Degradation of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b expressing soluble methane monooxygenase. Appl Environ Microbiol. 1989 Nov;55(11):2819–2826. doi: 10.1128/aem.55.11.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzer K. P., Buchholz L. A., Lidstrom M. E., Remsen C. C. Separation of methanotrophic bacteria by using percoll and its application to isolation of mixed and pure cultures. Appl Environ Microbiol. 1991 Dec;57(12):3656–3659. doi: 10.1128/aem.57.12.3656-3659.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. I., Colby J., Dalton H. A comparison of the substrate and electron-donor specificities of the methane mono-oxygenases from three strains of methane-oxidizing bacteria. Biochem J. 1979 Jan 1;177(1):361–364. doi: 10.1042/bj1770361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Brusseau G. A., Hanson R. S., Waclett L. P. Biodegradation of trichloroethylene by Methylosinus trichosporium OB3b. Appl Environ Microbiol. 1989 Dec;55(12):3155–3161. doi: 10.1128/aem.55.12.3155-3161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Hanson R. S. Soluble methane monooxygenase component B gene probe for identification of methanotrophs that rapidly degrade trichloroethylene. Appl Environ Microbiol. 1992 Mar;58(3):953–960. doi: 10.1128/aem.58.3.953-960.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Gibson D. T. Rapid method for detection and quantitation of hydroxylated aromatic intermediates produced by microorganisms. Appl Environ Microbiol. 1983 Mar;45(3):1144–1147. doi: 10.1128/aem.45.3.1144-1147.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. T., Wilson B. H. Biotransformation of trichloroethylene in soil. Appl Environ Microbiol. 1985 Jan;49(1):242–243. doi: 10.1128/aem.49.1.242-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]