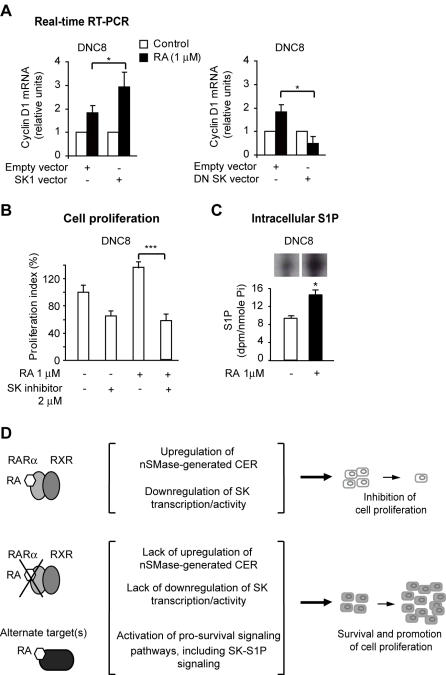
Figure 8. SK1-S1P signaling: a candidate growth promoting mechanism of non-RAR-mediated RA action.
A) Transient exogenous expression of SK1 in DNC8 cells leads to upregulation of cyclin D1 transcription relative to cells expressing the cognate empty vector (left). Conversely, transient exogenous expression of a dominant negative SK mutant in DNC8 cells negatively affects RA-induced cyclin D1 transcription (right). B) RA-induced DNC8 proliferation is significantly decreased by treatment with a SK inhibitor. C) RA upregulates the S1P level (spots in the upper insert) in DNC8 cells. D) Scheme showing that RA action mediated through RARα results in upregulation of nSMase-generated CER sythesis, concomitant with downregulation of SK1 transcription/activity. These concerted antiproliferative metabolic changes concur to inhibit cell proliferation. Consequent to an impaired RA-RARα signaling, these concerted antiproliferative metabolic changes do not occur, thus cells survive in the presence of RA. Moreover, RA, through alternate, non-RAR (genomic or non-genomic) target(s), activates pro-survival signaling pathways, including the SK signaling pathway, thus leading to the expansion of the RA-resistant cell pool.