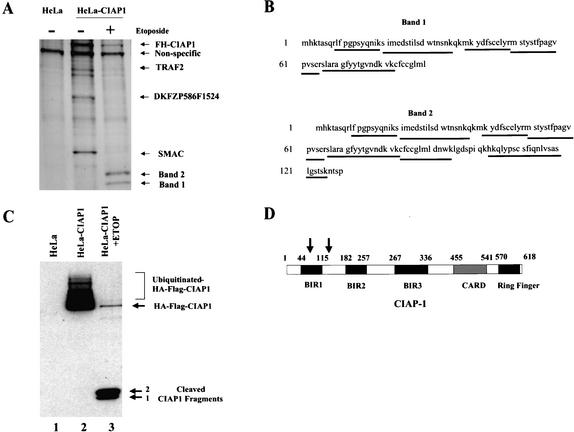
Figure 2.
CIAP1 is cleaved in HeLa cells upon etoposide treatment. (A) Silver stain analysis of proteins immunoprecipitated from cell lysates of control untreated HeLa cells (lane 1), untreated HeLa-CIAP1 cells (lane 2) or etoposide-treated HeLa-CIAP1 cells (lane 3). The proteins were identified by MALDI-quadrupole ion trap mass spectrometric analysis using both MS and MS/MS modes. (B) MALDI-quadruple mass spectrometric analysis of the two small proteins purified from etoposide-treated HeLa-CIAP1 cell lysate (as indicated in A). Underlined sequences are tryptic peptides identified from each protein, all mapping to the N-terminal CIAP1 sequence. (C) Immunoprecipitated proteins from cell lysates of control untreated HeLa cells (lane 1), untreated HeLa-CIAP1 cells (lane 2), or etoposide-treated HeLa-CIAP1 cells (lane 3), analyzed by immunoblotting with anti-HA antibody. (D) Domain structure of CIAP1 and the approximate cleavage sites, as indicated by arrows. BIR, birculoviral IAP Repeat domain; CARD, caspase recruitment domain; Ring Finger domain.