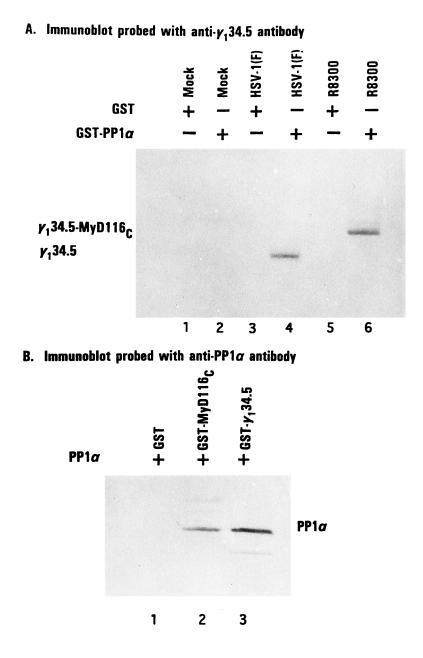
Figure 1.
Association of PP1α with γ134.5 and MyD116. (A) Replicate HeLa cell cultures were harvested in lysis buffer containing 10 mM Hepes (pH 7.6), 250 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, and 2 mM benzamidine 18 hr after mock infection or infection with 10 pfu of HSV-1(F) or R8300 per cell. After 30 min on wet ice and low speed centrifugation to remove nuclei, the supernatant fluids were precleared with GST beads and then reacted with GST phosphatase 1 bound beads at 4°C overnight. After extensive rinsing, the proteins bound to beads were solubilized by boiling in disruption buffer containing 50 mM Tris·HCl (pH 7.0), 5% 2-mercaptoethanol, 2% SDS, and 2.75% sucrose, electrophoretically separated on denaturing 12% polyacrylamide gels, and transferred to a nitrocellulose sheet, and the blot was probed with anti-γ134.5 serum (1). The positions of γ134.5 protein and of the chimeric protein γ134.5–MyD116 are shown. (B) An aliquot of GST–PP1α fusion protein bound to beads was reacted with 25 units of thrombin (Sigma) in PBS at room temperature. After 8 hr, the mixture was spun in a table top centrifuge and the supernatant fluid containing PP1α was then dialyzed against lysis buffer, reacted with GST, GST–MyD116C, or GST–γ134.5C bound to beads and processed as described in A. PP1 was detected with antiphosphatase 1α antibody (Upstate Biotechnology, Lake Placid, NY).