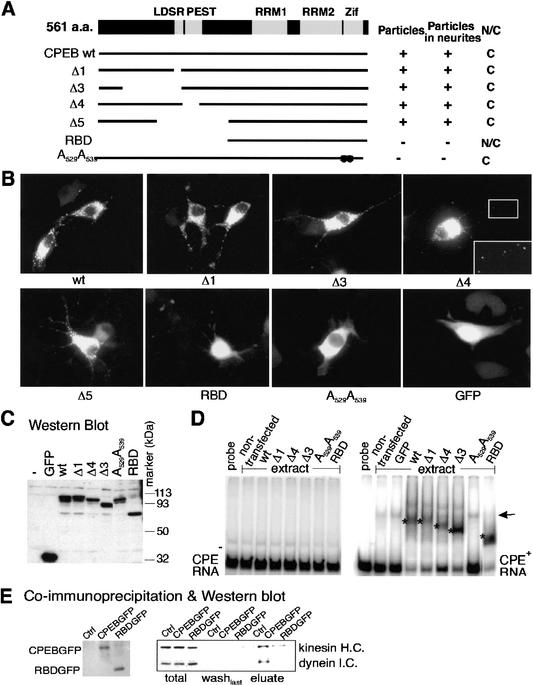
Figure 6.
A CPEB mutant is defective for kinesin and dynein interaction. (A) Salient features of CPEB showing the LDSR aurora phosphorylation site, a PEST instability region, two RNA recognition motifs (RRMs) and two zinc fingers (Zif). The structures of various CPEB mutant proteins are also shown, as well as whether they form granules, are transported to neurites, or are nuclear or cytoplasmic. (B) Localization of wild-type and mutant CPEB-GFP fusion proteins in transfected B104 cells. (C) Western blot of wild-type and mutant CPEB-GFP proteins in transfected B104 cells. (D) Gel shift using extracts from B104 cells transfected with wild-type or mutant CPEB-GFP fusion proteins with CPE-lacking (left) or CPE-containing (right) RNA. The asterisks denote specific shifts attributed to CPEB proteins. The arrow denotes a nonspecific band caused by an unknown protein from B104 cells. (E) Coimmunoprecipitation of wild-type or RBD mutant CPEB-GFP fusion proteins with motor proteins. Cells infected with DNA encoding the heterologous proteins noted above were immunoprecipitated, washed, and eluted with SDS loading buffer. The eluates were probed for CPEB-GFP and RBD-GFP (left) as well as kinesin heavy chain and dynein intermediate chain.