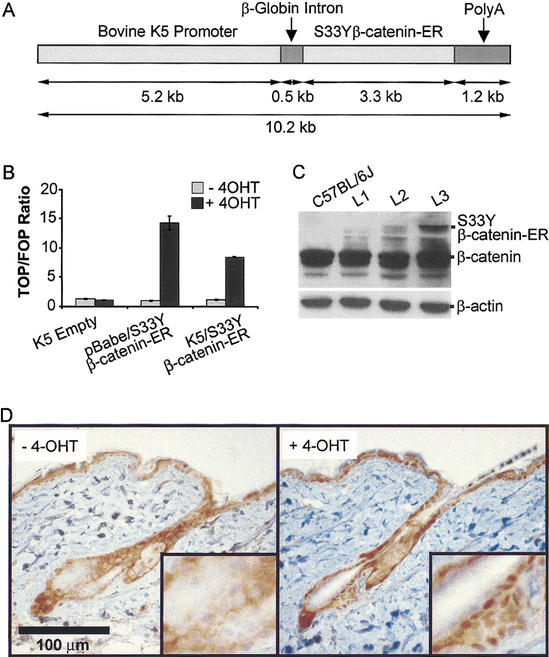
Figure 1.
Activation of the K5/S33Yβ-catenin–ER protein by 4-OHT and expression in transgenic mice. (A) Schematic of the K5/S33Yβ-catenin–ER construct. A cDNA encoding the chimeric protein was subcloned into a bovine K5 expression cassette. (B) Activity of the K5/S33Yβ-catenin–ER protein is inducible by 4-OHT in 1811 keratinocytes. The 1811 keratinocytes were transfected with an empty K5 cassette, the pBabe/S33Yβ-catenin–ER construct, or the K5/S33Yβ-catenin–ER construct along with either the TCF-responsive reporter construct TOPFLASH or control FOPFLASH construct. Cells were then treated with either 4-OHT in ethanol, or ethanol alone, and harvested 30 h later to assess luciferase activity. The assays were performed in duplicate; data are reported as the ratio of relative light units for TOPFLASH:FOPFLASH, normalized for transfection efficiency. (C) Expression of the K5/S33Yβ-catenin–ER fusion protein relative to endogenous β-catenin in transgenic mouse lines. Protein was isolated from tail skin of F1 mice derived from three independently derived founders and subjected to Western blot analysis with a mouse monoclonal anti-β-catenin antibody. The blot was reprobed with an anti-β-actin antibody to verify equal loading and transfer. (D) β-catenin protein localization in a clipped region of dorsal skin from K5/S33Yβ-catenin–ER transgenic mice not treated (−4-OHT) or 24 h after treatment with a single topical dose of 4-OHT (+4-OHT).