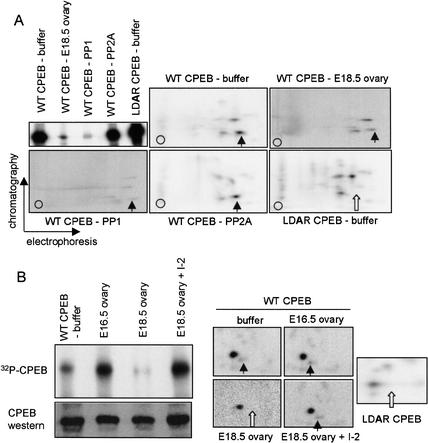
Figure 4.
Phosphatase activity in E18.5 ovaries. (A) Recombinant histidine-tagged CPEB, containing a wild-type or mutated (LDAR) aurora phosphorylation site, was incubated in a Xenopus egg extract together with γ32P-ATP. The CPEB proteins were then isolated on a nickel-agarose column and incubated with buffer alone, phosphatase PP1, phosphatase PP2A, or an extract derived from E18.5 wild-type ovaries. When analyzed by quantitative SDS-PAGE, only PP1 and the E18.5 ovary extract had the capability of dephosphorylating CPEB. All phosphorylated CPEB proteins were subsequently analyzed by qualitative two-dimensional phospho-peptide mapping. In each panel, the vertical arrow denotes the aurora-catalyzed phospho-peptide, which was absent from the mutant CPEB (white arrow). The signals from the panels denoted E18.5 ovary and PP1 were enhanced to show the individual phospho-peptides, which otherwise would not be detected at the same exposure level as in the other three panels. (B) Phospho-CPEB was added to E16.5 and E18.5 ovary extracts, as well as E18.5 ovary extracts that were preincubated with I-2, a specific inhibitor of PP1. The degree of CPEB dephosphorylation was then monitored by SDS-PAGE and autoradiography, as well as two-dimensional phospho-peptide mapping (arrows are as noted above). Some of the extract was also Western blotted and probed for CPEB.