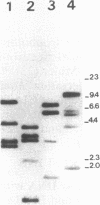
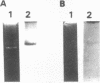
Abstract
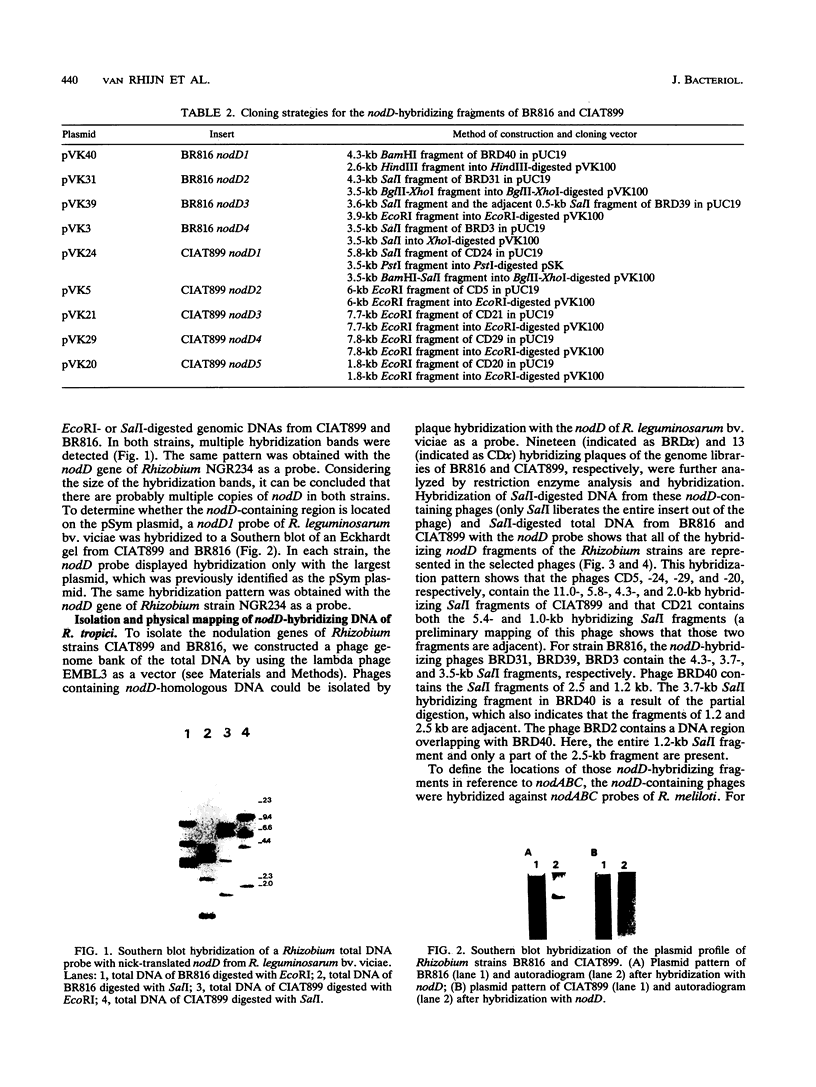
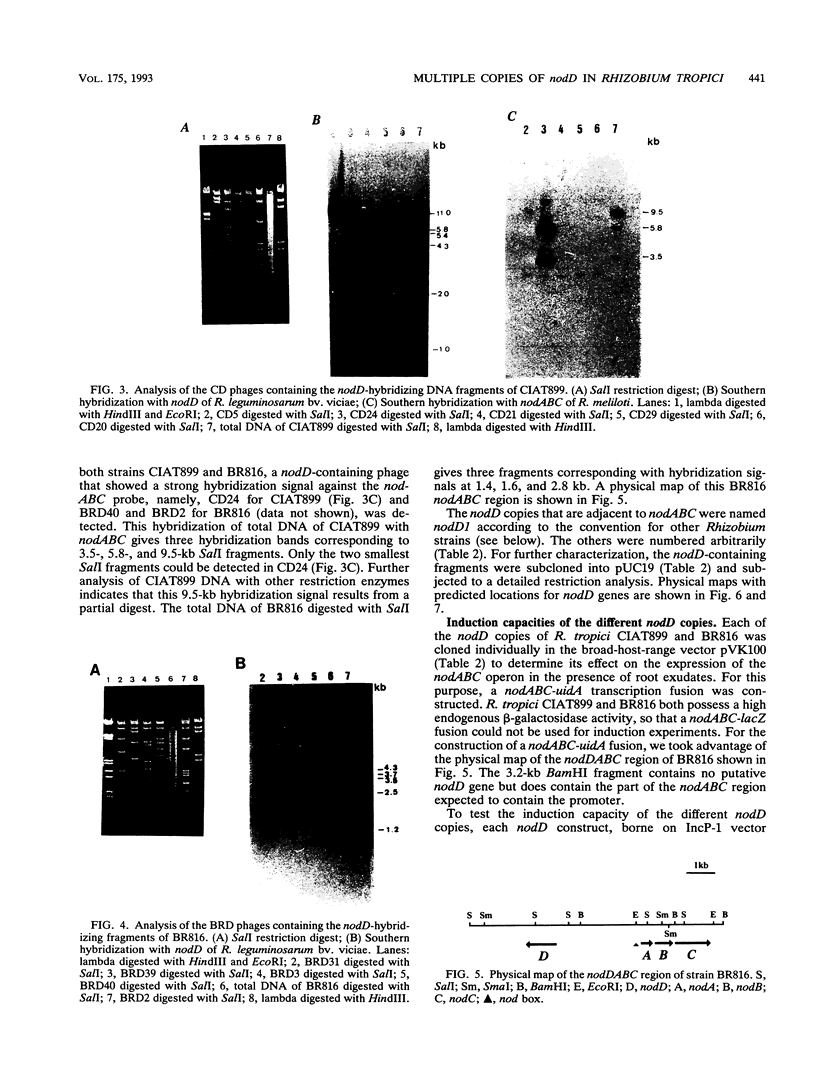
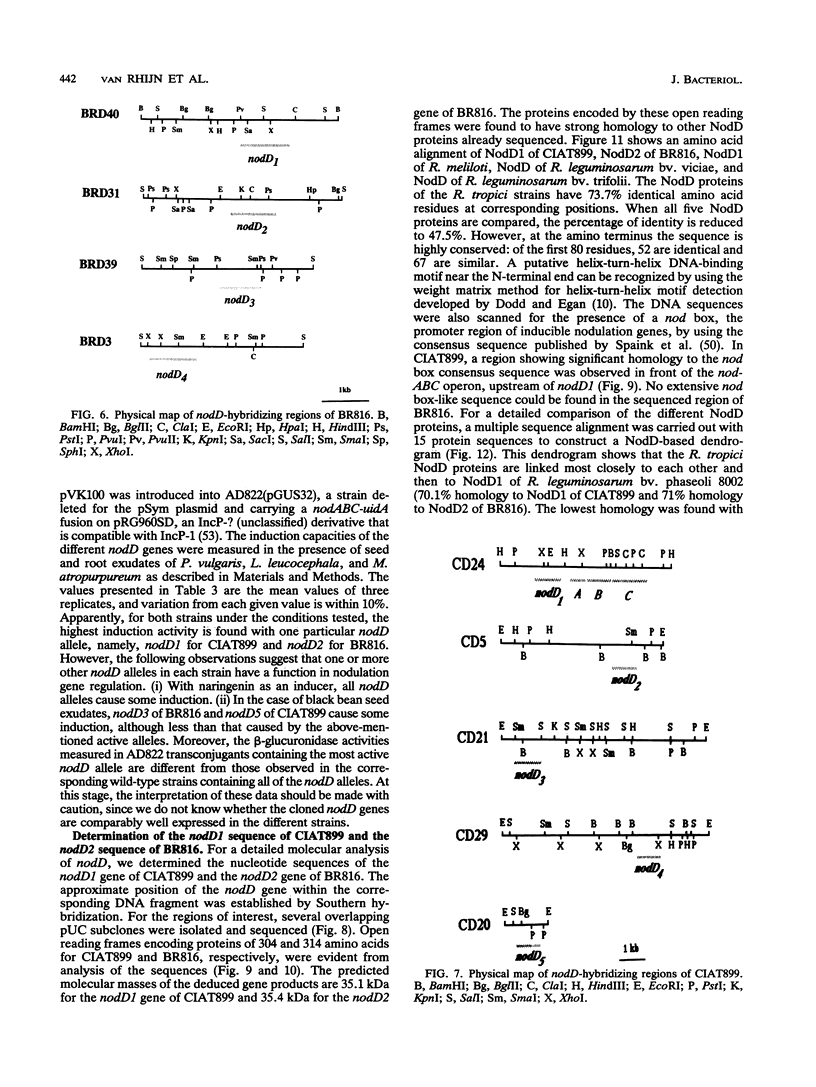
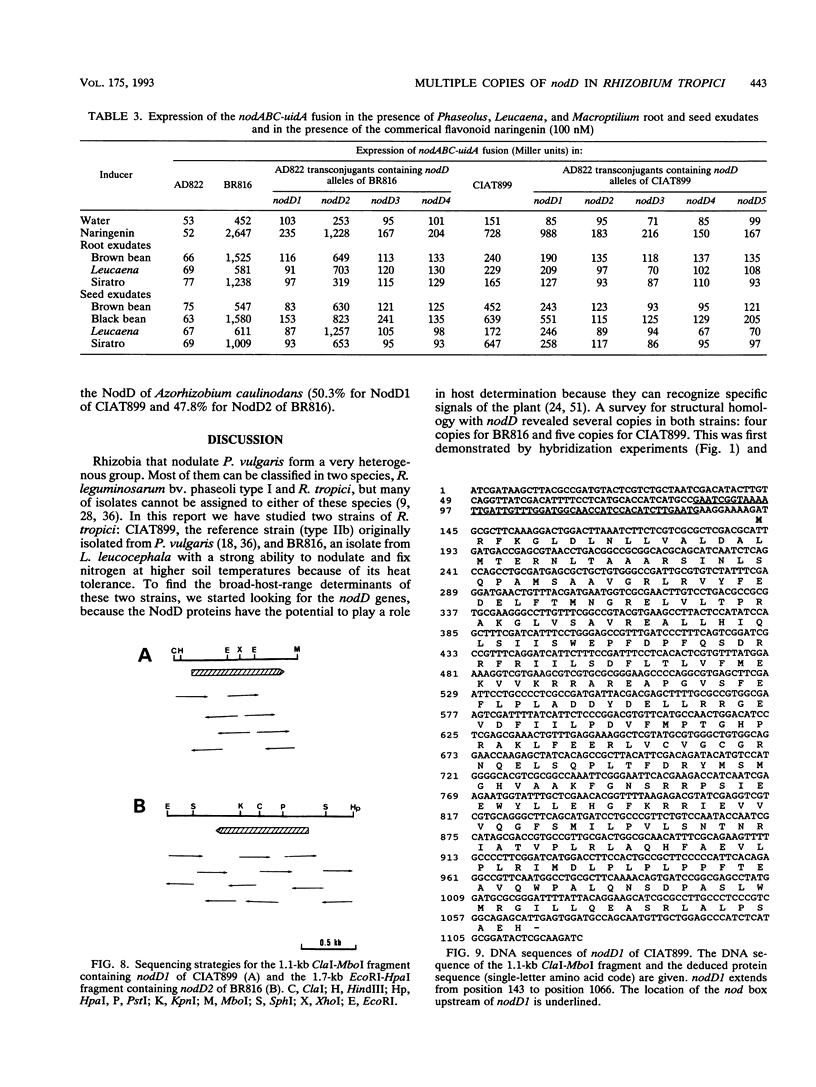
Rhizobium tropici strains are able to nodulate a wide range of host plants: Phaseolus vulgaris, Leucaena spp., and Macroptilium atropurpureum. We studied the nodD regulatory gene for nodulation of two R. tropici strains: CIAT899, the reference R. tropici type IIb strain, and BR816, a heat-tolerant strain isolated from Leucaena leucocephala. A survey revealed several nodD-hybridizing DNA regions in both strains: five distinct regions in CIAT899 and four distinct regions in BR816. Induction experiments of a nodABC-uidA fusion in combination with different nodD-hybridizing fragments in the presence of root exudates of the different hosts indicate that one particular nodD copy contributes to nodulation gene induction far more than any other nodD copy present. The nucleotide sequences of both nodD genes are reported here and show significant homology to those of the nodD genes of other rhizobia and a Bradyrhizobium strain. A dendrogram based on the protein sequences of 15 different NodD proteins shows that the R. tropici NodD proteins are linked most closely to each other and then to the NodD of Rhizobium phaseoli 8002.
Full text
PDF


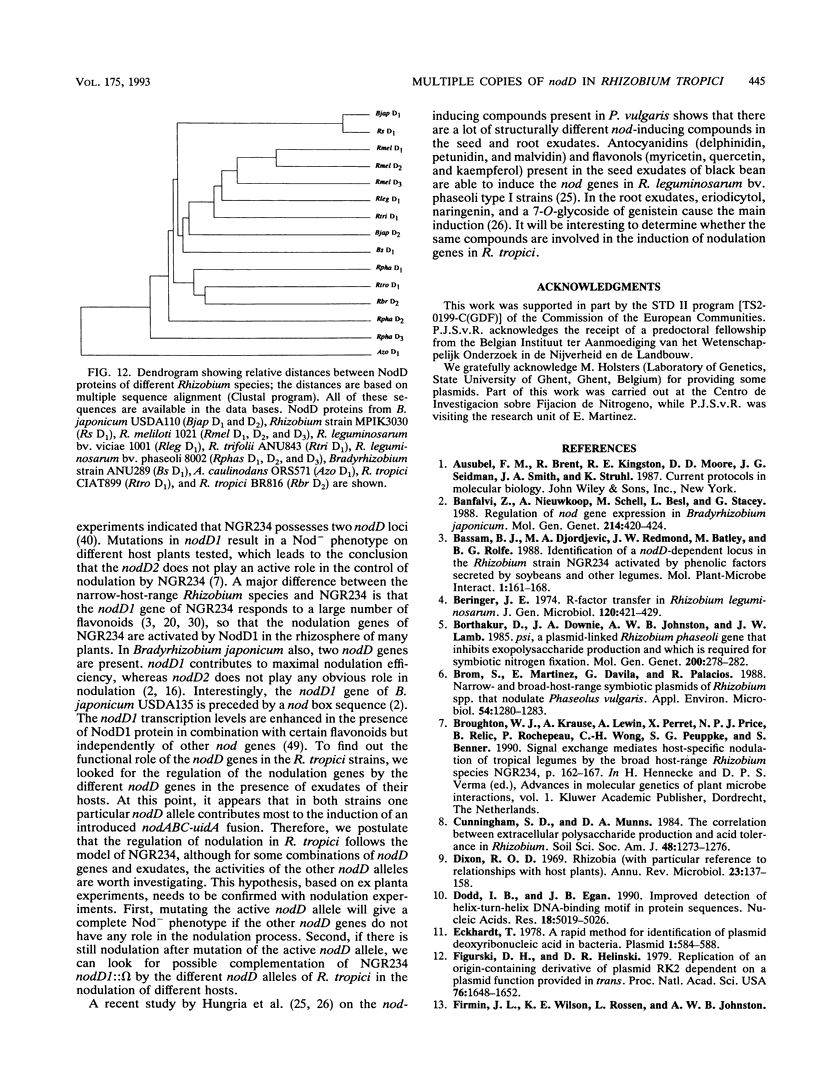


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banfalvi Z., Nieuwkoop A., Schell M., Besl L., Stacey G. Regulation of nod gene expression in Bradyrhizobium japonicum. Mol Gen Genet. 1988 Nov;214(3):420–424. doi: 10.1007/BF00330475. [DOI] [PubMed] [Google Scholar]
- Bassam B. J., Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Identification of a nodD-dependent locus in the Rhizobium strain NGR234 activated by phenolic factors secreted by soybeans and other legumes. Mol Plant Microbe Interact. 1988 Apr;1(4):161–168. doi: 10.1094/mpmi-1-161. [DOI] [PubMed] [Google Scholar]
- Brom S., Martinez E., Dávila G., Palacios R. Narrow- and Broad-Host-Range Symbiotic Plasmids of Rhizobium spp. Strains That Nodulate Phaseolus vulgaris. Appl Environ Microbiol. 1988 May;54(5):1280–1283. doi: 10.1128/aem.54.5.1280-1283.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Rhizobia (with particular reference to relationships with host plants). Annu Rev Microbiol. 1969;23:137–158. doi: 10.1146/annurev.mi.23.100169.001033. [DOI] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990 Sep 11;18(17):5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. F., Egelhoff T. T., Mulligan J. T., Long S. R. Specific binding of proteins from Rhizobium meliloti cell-free extracts containing NodD to DNA sequences upstream of inducible nodulation genes. Genes Dev. 1988 Mar;2(3):282–293. doi: 10.1101/gad.2.3.282. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Göttfert M., Holzhäuser D., Bäni D., Hennecke H. Structural and functional analysis of two different nodD genes in Bradyrhizobium japonicum USDA110. Mol Plant Microbe Interact. 1992 May-Jun;5(3):257–265. doi: 10.1094/mpmi-5-257. [DOI] [PubMed] [Google Scholar]
- Göttfert M., Horvath B., Kondorosi E., Putnoky P., Rodriguez-Quiñones F., Kondorosi A. At least two nodD genes are necessary for efficient nodulation of alfalfa by Rhizobium meliloti. J Mol Biol. 1986 Oct 5;191(3):411–420. doi: 10.1016/0022-2836(86)90136-1. [DOI] [PubMed] [Google Scholar]
- Honma M. A., Asomaning M., Ausubel F. M. Rhizobium meliloti nodD genes mediate host-specific activation of nodABC. J Bacteriol. 1990 Feb;172(2):901–911. doi: 10.1128/jb.172.2.901-911.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M. A., Ausubel F. M. Rhizobium meliloti has three functional copies of the nodD symbiotic regulatory gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8558–8562. doi: 10.1073/pnas.84.23.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B., Bachem C. W., Schell J., Kondorosi A. Host-specific regulation of nodulation genes in Rhizobium is mediated by a plant-signal, interacting with the nodD gene product. EMBO J. 1987 Apr;6(4):841–848. doi: 10.1002/j.1460-2075.1987.tb04829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungria M., Joseph C. M., Phillips D. A. Anthocyanidins and Flavonols, Major nod Gene Inducers from Seeds of a Black-Seeded Common Bean (Phaseolus vulgaris L.). Plant Physiol. 1991 Oct;97(2):751–758. doi: 10.1104/pp.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungria M., Joseph C. M., Phillips D. A. Rhizobium nod Gene Inducers Exuded Naturally from Roots of Common Bean (Phaseolus vulgaris L.). Plant Physiol. 1991 Oct;97(2):759–764. doi: 10.1104/pp.97.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Martínez-Romero E., Segovia L., Mercante F. M., Franco A. A., Graham P., Pardo M. A. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Bacteriol. 1991 Jul;41(3):417–426. doi: 10.1099/00207713-41-3-417. [DOI] [PubMed] [Google Scholar]
- Martínez E., Palacios R., Sánchez F. Nitrogen-fixing nodules induced by Agrobacterium tumefaciens harboring Rhizobium phaseoli plasmids. J Bacteriol. 1987 Jun;169(6):2828–2834. doi: 10.1128/jb.169.6.2828-2834.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. A family of activator genes regulates expression of Rhizobium meliloti nodulation genes. Genetics. 1989 May;122(1):7–18. doi: 10.1093/genetics/122.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitz J., Jacobs G. H. Polymorphism of the long-wavelength cone in normal human colour vision. Nature. 1986 Oct 16;323(6089):623–625. doi: 10.1038/323623a0. [DOI] [PubMed] [Google Scholar]
- Perret X., Broughton W. J., Brenner S. Canonical ordered cosmid library of the symbiotic plasmid of Rhizobium species NGR234. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1923–1927. doi: 10.1073/pnas.88.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Rostas K., Kondorosi E., Horvath B., Simoncsits A., Kondorosi A. Conservation of extended promoter regions of nodulation genes in Rhizobium. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1757–1761. doi: 10.1073/pnas.83.6.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaman H. R., Okker R. J., Lugtenberg B. J. Regulation of nodulation gene expression by NodD in rhizobia. J Bacteriol. 1992 Aug;174(16):5177–5182. doi: 10.1128/jb.174.16.5177-5182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., John M., Kondorosi E., Kondorosi A., Wieneke U., Schröder G., Schröder J., Schell J. Mapping of the protein-coding regions of Rhizobium meliloti common nodulation genes. EMBO J. 1984 Aug;3(8):1705–1711. doi: 10.1002/j.1460-2075.1984.tb02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Puvanesarajah V., Carlson R. W., Barbour W. M., Stacey G. Bradyrhizobium japonicum nodD1 can be specifically induced by soybean flavonoids that do not induce the nodYABCSUIJ operon. J Biol Chem. 1992 Jan 5;267(1):310–318. [PubMed] [Google Scholar]
- Van den Eede G., Deblaere R., Goethals K., Van Montagu M., Holsters M. Broad host range and promoter selection vectors for bacteria that interact with plants. Mol Plant Microbe Interact. 1992 May-Jun;5(3):228–234. doi: 10.1094/mpmi-5-228. [DOI] [PubMed] [Google Scholar]
- Vargas C., Martinez L. J., Megias M., Quinto C. Identification and cloning of nodulation genes and host specificity determinants of the broad host-range Rhizobium leguminosarum biovar phaseoli strain CIAT899. Mol Microbiol. 1990 Nov;4(11):1899–1910. doi: 10.1111/j.1365-2958.1990.tb02039.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zaat S. A., Wijffelman C. A., Spaink H. P., van Brussel A. A., Okker R. J., Lugtenberg B. J. Induction of the nodA promoter of Rhizobium leguminosarum Sym plasmid pRL1JI by plant flavanones and flavones. J Bacteriol. 1987 Jan;169(1):198–204. doi: 10.1128/jb.169.1.198-204.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]