Abstract
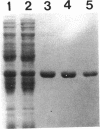
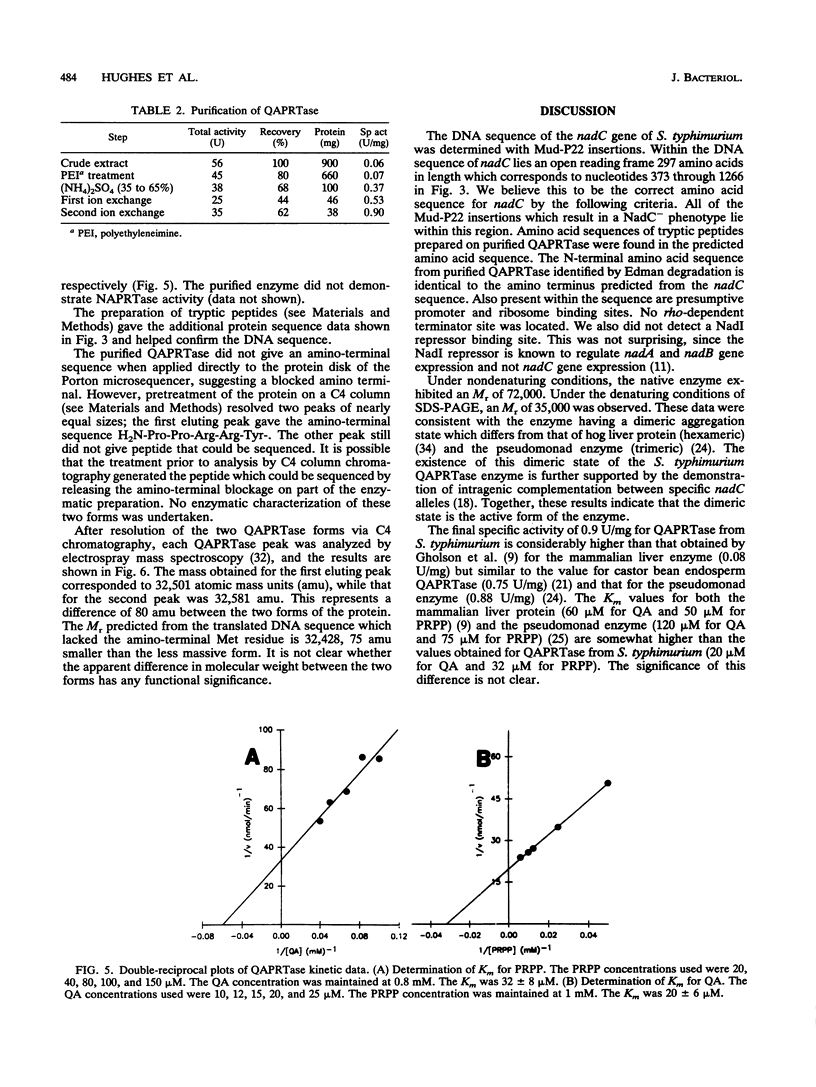
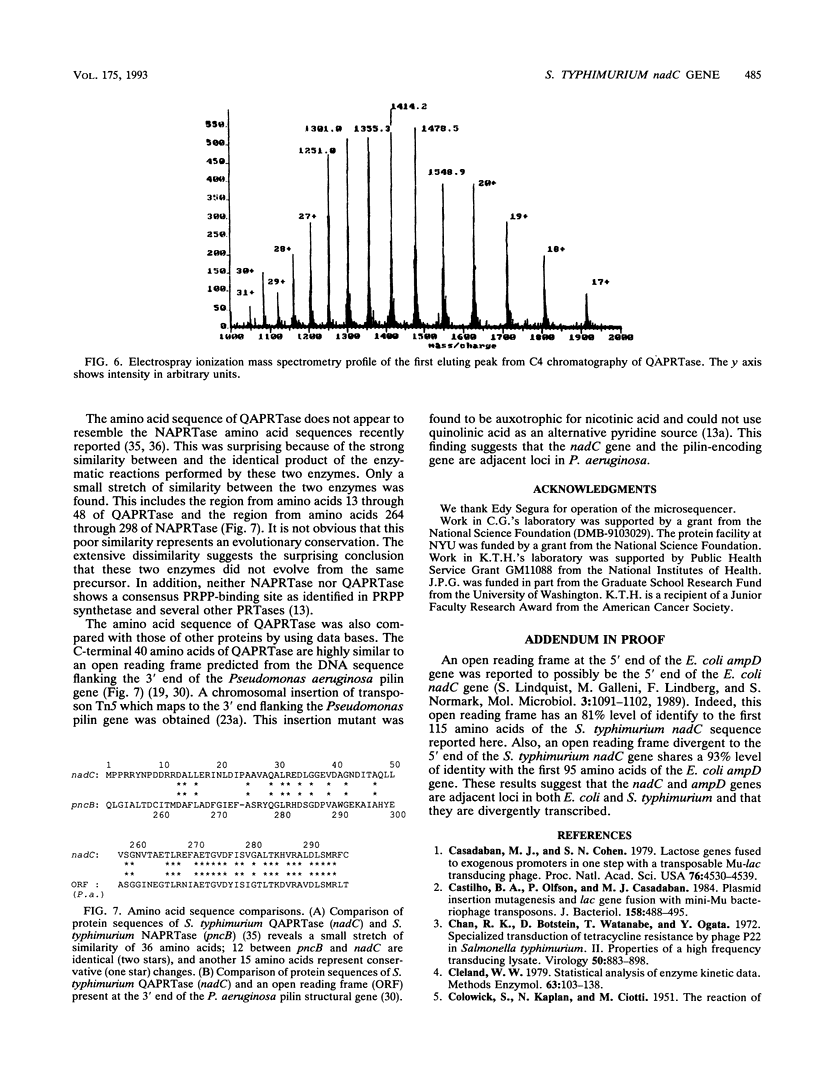
The Salmonella typhimurium nadC gene and its product, quinolinic acid phosphoribosyltransferase (QAPRTase), were characterized at the molecular and biochemical levels. Fusions of Mud-lac elements isolated in the nadC gene were converted to Mud-P22 insertions. Starting with six original Mud-lac fusions, the entire sequence of the nadC gene was readily obtained. The sequence shows a long open reading frame with two potential initiator methionines, one of which is preceded by the Shine-Dalgarno sequence GGAG-7-nucleotide-ATG. The protein predicted from this second open reading frame is 297 residues in length. The nadC gene was subcloned into a T7-based expression system, allowing for facile purification of the QAPRTase (EC 2.4.2.19) protein to homogeneity. Upon gel filtration, the protein gave an M(r) of 72,000, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis gave a subunit M(r) of 35,000. Automated Edman degradation of several tryptic peptides confirmed the amino acid sequence predicted from the DNA sequence. Chromatography of the apparently homogeneous enzyme on reverse-phase high-performance liquid chromatography resolved two protein species. One of these species failed to give an amino-terminal sequence, while the other yielded the amino-terminal sequence predicted by the second open reading frame and lacked the initiator methionine. The mass of the mature protein, predicted from its DNA sequence, was 32,428 Da. Electrospray mass spectrometry gave masses of 32,501 and 32,581 Da for the two peptides. Steady-state kinetics on the purified QAPRTase indicated Km values of 32 microM for 5-phosphoribosyl-1-pyrophosphate and 20 microM for quinolinate. Vmax was 0.9 U/mg, similar to values reported for this enzyme by other sources.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Botstein D., Watanabe T., Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology. 1972 Dec;50(3):883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Cookson B. T., Olivera B. M., Roth J. R. Genetic characterization and regulation of the nadB locus of Salmonella typhimurium. J Bacteriol. 1987 Sep;169(9):4285–4293. doi: 10.1128/jb.169.9.4285-4293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Holley-Guthrie E. A., Warren F. Regulation of NAD metabolism in Salmonella typhimurium: genetic analysis and cloning of the nadR repressor locus. Mol Gen Genet. 1987 Jun;208(1-2):279–287. doi: 10.1007/BF00330454. [DOI] [PubMed] [Google Scholar]
- Gholson R. K., Tritz G. J., Matney T. S., Andreoli A. J. Mode of nicotinamide adenine dinucleotide utilization by Escherichia coli. J Bacteriol. 1969 Sep;99(3):895–896. doi: 10.1128/jb.99.3.895-896.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmiel S. P., Snavely M. D., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J Bacteriol. 1986 Dec;168(3):1444–1450. doi: 10.1128/jb.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley E. A., Foster J. W. Bacteriophage P22 as a vector for Mu mutagenesis in Salmonella typhimurium: isolation of nad-lac and pnc-lac gene fusions. J Bacteriol. 1982 Nov;152(2):959–962. doi: 10.1128/jb.152.2.959-962.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley E. A., Spector M. P., Foster J. W. Regulation of NAD biosynthesis in Salmonella typhimurium: expression of nad-lac gene fusions and identification of a nad regulatory locus. J Gen Microbiol. 1985 Oct;131(10):2759–2770. doi: 10.1099/00221287-131-10-2759. [DOI] [PubMed] [Google Scholar]
- Hove-Jensen B., Harlow K. W., King C. J., Switzer R. L. Phosphoribosylpyrophosphate synthetase of Escherichia coli. Properties of the purified enzyme and primary structure of the prs gene. J Biol Chem. 1986 May 25;261(15):6765–6771. [PubMed] [Google Scholar]
- Hughes K. T., Olivera B. M., Roth J. R. Rec dependence of mu transposition from P22-transduced fragments. J Bacteriol. 1987 Jan;169(1):403–409. doi: 10.1128/jb.169.1.403-409.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Directed formation of deletions and duplications using Mud(Ap, lac). Genetics. 1985 Feb;109(2):263–282. doi: 10.1093/genetics/109.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R., Olivera B. M. A genetic characterization of the nadC gene of Salmonella typhimurium. Genetics. 1991 Apr;127(4):657–670. doi: 10.1093/genetics/127.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988 May;119(1):9–12. doi: 10.1093/genetics/119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K., Parker M. L., Lory S. Nucleotide sequence and transcriptional initiation site of two Pseudomonas aeruginosa pilin genes. J Biol Chem. 1986 Nov 25;261(33):15703–15708. [PubMed] [Google Scholar]
- Lindquist S., Galleni M., Lindberg F., Normark S. Signalling proteins in enterobacterial AmpC beta-lactamase regulation. Mol Microbiol. 1989 Aug;3(8):1091–1102. doi: 10.1111/j.1365-2958.1989.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Liu S. L., Sanderson K. E. A physical map of the Salmonella typhimurium LT2 genome made by using XbaI analysis. J Bacteriol. 1992 Mar;174(5):1662–1672. doi: 10.1128/jb.174.5.1662-1672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. F., Byerrum R. U. Quinolinic acid phosphoribosyltransferase from castor bean endosperm. I. Purification and characterization. J Biol Chem. 1974 Nov 10;249(21):6817–6823. [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Musick W. D. Structural features of the phosphoribosyltransferases and their relationship to the human deficiency disorders of purine and pyrimidine metabolism. CRC Crit Rev Biochem. 1981;11(1):1–34. doi: 10.3109/10409238109108698. [DOI] [PubMed] [Google Scholar]
- Packman P. M., Jakoby W. B. Crystalline quinolinate phosphoribosyltransferase. II. Properties of the enzyme. J Biol Chem. 1967 May 10;242(9):2075–2079. [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry P. A., Finlay B. B., Pasloske B. L., Paranchych W., Pearlstone J. R., Smillie L. B. Comparative studies of the amino acid and nucleotide sequences of pilin derived from Pseudomonas aeruginosa PAK and PAO. J Bacteriol. 1985 Nov;164(2):571–577. doi: 10.1128/jb.164.2.571-577.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton R. E., Rocha V., Rosser R. J., Andreoli A. J., Shimoyama M., Kosaka A., Chandler J. L., Gholson R. K. A comparative study of the regulation of nicotinamide-adenine dinucleotide biosynthesis. Biochim Biophys Acta. 1968 Feb 1;156(1):77–84. doi: 10.1016/0304-4165(68)90106-2. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Loo J. A., Edmonds C. G., Barinaga C. J., Udseth H. R. New developments in biochemical mass spectrometry: electrospray ionization. Anal Chem. 1990 May 1;62(9):882–899. doi: 10.1021/ac00208a002. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Taguchi H., Iwai K. Chemical modifications of the crystalline quinolinate phosphoribosyltransferase from hog liver. Biochim Biophys Acta. 1976 Jan 23;422(1):29–37. doi: 10.1016/0005-2744(76)90005-x. [DOI] [PubMed] [Google Scholar]
- Vinitsky A., Teng H., Grubmeyer C. T. Cloning and nucleic acid sequence of the Salmonella typhimurium pncB gene and structure of nicotinate phosphoribosyltransferase. J Bacteriol. 1991 Jan;173(2):536–540. doi: 10.1128/jb.173.2.536-540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubbolts M. G., Terpstra P., van Beilen J. B., Kingma J., Meesters H. A., Witholt B. Variation of cofactor levels in Escherichia coli. Sequence analysis and expression of the pncB gene encoding nicotinic acid phosphoribosyltransferase. J Biol Chem. 1990 Oct 15;265(29):17665–17672. [PubMed] [Google Scholar]
- Youderian P., Sugiono P., Brewer K. L., Higgins N. P., Elliott T. Packaging specific segments of the Salmonella chromosome with locked-in Mud-P22 prophages. Genetics. 1988 Apr;118(4):581–592. doi: 10.1093/genetics/118.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Olivera B. M., Roth J. R. Genetic characterization of the pnuC gene, which encodes a component of the nicotinamide mononucleotide transport system in Salmonella typhimurium. J Bacteriol. 1989 Aug;171(8):4402–4409. doi: 10.1128/jb.171.8.4402-4409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]