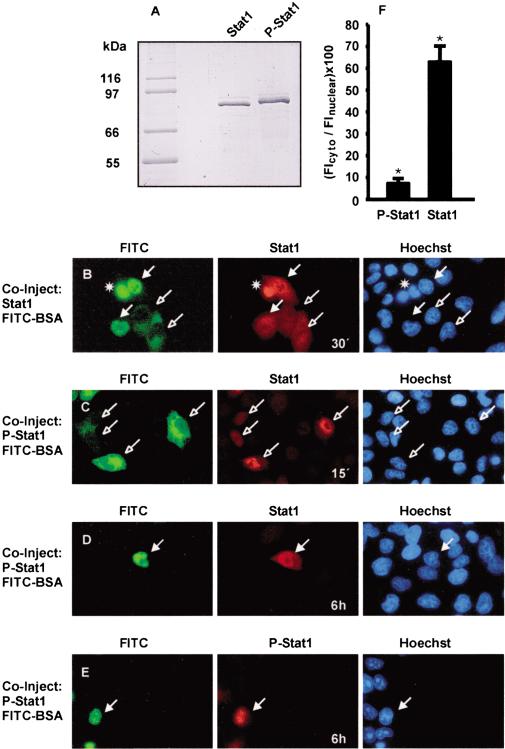
Figure 1.
Nucleocytoplasmic shuttling and nuclear accumulation of Stat1 in resting cells. HeLa cells were comicroinjected with unphosphorylated (Stat1) or tyrosine-phosphorylated (P-Stat1) Stat1 and FITC-BSA. After incubation for the indicated times and fixation of the cells, immunocytochemistry and staining of nuclei with Hoechst dye followed. Injected cells are indicated with filled (nuclear injection) or unfilled (cytoplasmic injection) arrows. (A) SDS-PAGE analysis of the Stat1 preparations (1 μg each) used in B–E. The migration of Coomassie-stained molecular size markers is indicated to the left. (B) Unphosphorylated Stat1 was injected into the nucleus or cytoplasm, and the cells were then incubated at 37°C for 30 min. Cells were stained with anti-Stat1 antibody. (*) Dead cell. (C) Tyrosine-phosphorylated Stat1 was injected into the cytoplasm, and the cells were subsequently incubated at 37°C for 15 min. Cells were stained with anti-Stat1 antibody. (D–F) Tyrosine-phosphorylated Stat1 was injected into the nucleus, and the cells were incubated at 37°C for 6 h before fixation. Immunocytochemistry was performed with an antibody recognizing Stat1 or P-Stat1. Representative cells are shown in D and E. For 12 cells of each, the ratio of cytoplasmic and nuclear Stat1 immunofluorescence density was determined and is depicted in the bar diagram (F). Statistically significant differences are indicated by *.