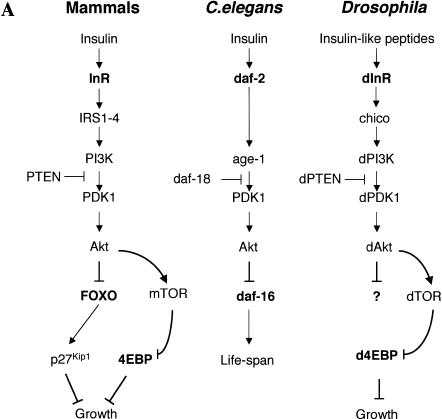
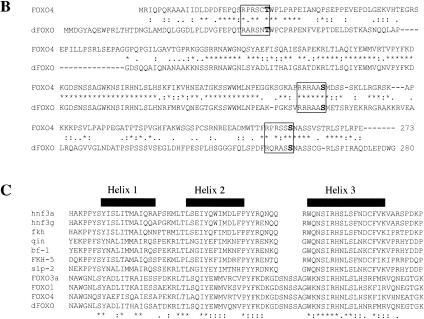
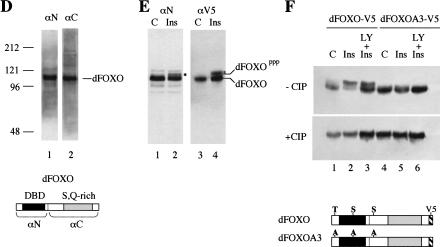
Figure 1.
(A) The insulin receptor signaling pathway is conserved in mammals, C. elegans, and Drosophila. (B) Identification of a Drosophila homolog of FOXO/DAF-16. Sequence alignment showing the high degree of conservation displayed in the DNA-binding domains of FOXO4 and dFOXO. The three conserved Akt phosphorylation motifs are boxed, and the amino acids that can be phosphorylated are in boldface. Asterisks mark identical amino acids; colons mark conserved amino acid changes; dots indicate weakly conserved changes. (C) FOXO family members have a 5-amino-acid insertion between helices 2 and 3 in the DNA-binding domain, which is lacking in the rest of forkhead-related proteins. (D) Antibodies raised against recombinant C- and N-terminal regions of dFOXO recognize a band with similar mobility in Drosophila S2 cell extracts. A representation of dFOXO indicating the fragments used for antibody production is shown below. (E) Insulin treatment produces a slower-mobility form of dFOXO (marked with an asterisk) that is detected with endogenous (lanes 1,2) or overexpressed (lanes 3,4) dFOXO. (F) Overexpressed dFOXO is phosphorylated upon insulin treatment of S2 cells (lane 2). Pretreatment of samples with LY294002 before insulin treatment reduces the amount of dFOXO that is phosphorylated (lane 3). dFOXOA3 is not phosphorylated upon insulin treatment (lanes 4–6). The lower panel shows the same samples after phosphatase treatment. A scheme representing wild-type and mutant dFOXO is shown below.