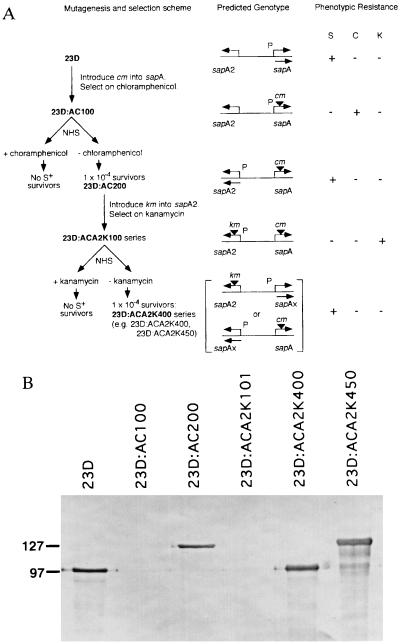
Figure 1.
Schematic representation of serial experiments to use km and cm cassettes to examine SLP gene rearrangement (A). Introduction of cm into strain 23D by marker rescue led to insertion into sapA to create 23D:AC100. This strain was selected on chloramphenicol-containing medium and, as expected, was chloramphenicol (C)-resistant, was S−, and not resistant to serum (S) and kanamycin (K), as previously reported (9). Incubation of 23D:AC100 with NHS selected for survivors (at a frequency of 1 × 10−4; AC200 series) that were S+ and serum-resistant but sensitive to kanamycin and chloramphenicol. Strain 23D:AC200, expressing sapA2, was further mutagenized by introduction of km into sapA2 to create the 23D:ACA2K100 series. These strains were S− and serum- and chloramphenicol-sensitive but kanamycin-resistant. Incubation with NHS selected for survivors (at a frequency of 1 × 10−4) that were S+ and serum-resistant but kanamycin- and chloramphenicol-sensitive (ACA2K400 series). The reciprocal relationship between antibiotic- and serum-resistance suggests that only a single promoter for SLP gene cassettes is present. All ACA2K400 series strains must have an SLP gene cassette other than sapA or sapA2 positioned downstream of the single SLP promoter. The predicted genotypes are depicted. P, sapA promoter; bent arrows, location and direction of transcription of SLP gene cassette; ▾, antibiotic resistance gene insertion. (B) Immunoblot of C. fetus strain 23D and selected mutants into which cm or cm and km were inserted into SLP gene cassettes. As expected, all the serum-resistant strains expressed SLP (of 97 or 127 kDa) recognized by antiserum to conserved C. fetus SLP determinants, whereas there was no expression for strains maintained on either antibiotic.