Scheme 1.
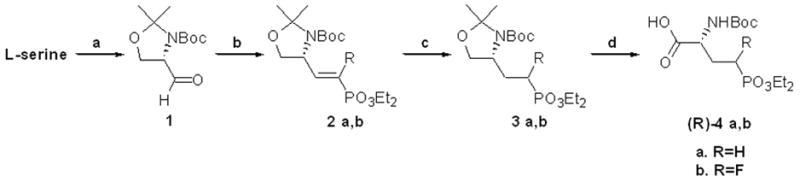
Synthesis of (3R)-4a,b – Method A: a.) i. SOCl2, MeOH, rt, 16h ii. Boc2O, Et3N, CH2Cl2, rt, 12h iii. 2,2-dimethoxypropane, p-TsOH, CH2Cl2, 0 °C to rt, 2h 62% (3 steps) iv. NaBH4, LiCl, 3:2 EtOH/THF, 0 °C to rt, 4h 89% v. DMSO, (COCl)2, CH2Cl2, −78 °C, then Et3N −78 °C to rt, 2–4 h, 97% b.) tetraethyl methylenebisphosphonate, n-BuLi, THF, −78 °C, rt, overnight, 75% (2a) or tetraethyl 2-fluoromethynebisphosphonate, n-BuLi, THF, -78 °C, rt, overnight, 31% (2b) c.) H2, Pd/C, rt., 12h, EtOH, 99% (3a) and 88% (3b) d.) Jones reagent, acetone, 0 °C to rt., 12h then, isopropyl alcohol, celite, rt, 15min., 59% (4a) and 48% (4b).
