Scheme 6.
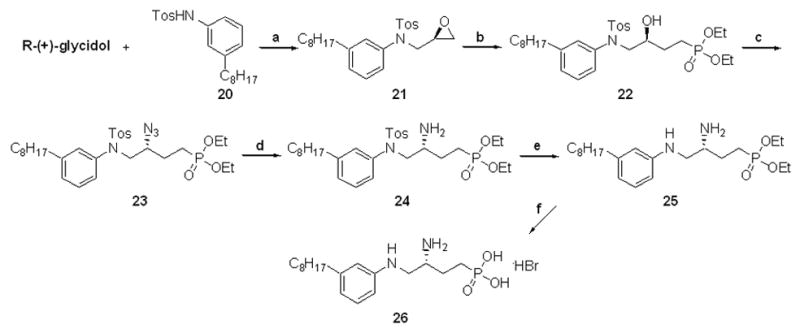
Synthesis of arylamine phosphonate analogue 26: a.) DIAD, PPh3, THF, 0 °C to rt, overnight, 69% b.) CH3PO3Et2, n-BuLi, BF3·OEt2, THF, –78 °C to rt, 2h then, NH4Cl, 2h, 97% c.) DPPA, DIAD, PPh3, CH2Cl2, 0 °C to rt, 20h, 85% (containing 5% OPPh3) d.) H2 (balloon), 20%w/w Pd(OH)2, 20:1 MeOH/conc. HCl, 1h, 100% e.)Na(s), NH3(l), –78 °C, 5 min.then EtOH, 25% (recovered 28% starting material) f.) TMSBr, CH2Cl2, rt, 4–6h then, 95:5 MeOH/H2O, 4h, rt, 95%.
