Figure 4.
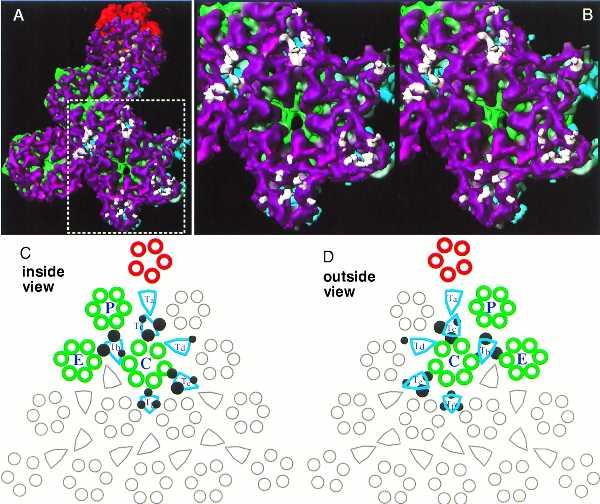
Contact points of scaffolding proteins on the capsid floor. The additional rod-like densities from the protease-minus capsid are super-imposed on the capsid shell of the wild-type B-capsid (A). For clarity, only those densities that are within a radial distance of ≈10 Å from the shell are shown. Panel B shows a stereo pair of an enlarged region surrounding the C hexon (indicated by the dotted rectangle in A). The schematic diagrams of one triangular face of the HSV capsid depict the points of contact of the additional mass densities on the floor as viewed from inside (C) and outside (D) of the capsid. All the morphological components in a triangular face are shown, and those components in one of the three asymmetric units are highlighted in color. Each asymmetric unit includes one-fifth of the penton (red), one each of P and C hexons (green), one-half an E hexon (green), one each of the triplexes Ta, Tb, Tc, Td, and Te, and one-third of the triplex Tf (aquamarine). Filled dark circles designate the points of contact of the scaffolding and the capsid shell. Larger circles represent more extensive contacts, and smaller circles indicate less extensive ones.
