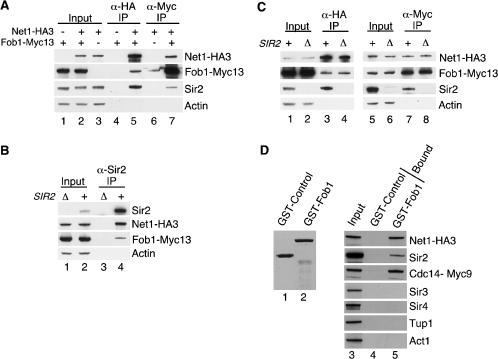
Figure 6.
The RENT complex physically associates with Fob1. (A) Western blots showing that Net1–HA3 coprecipitates with Sir2 and Fob1–Myc13 (lane 5) from whole-cell extracts. Fob1–Myc13 also coprecipitates Net1–HA3 and Sir2 (lane 7). Actin serves as a loading control. (–) Untagged; (+) tagged. One percent of whole-cell extract (input) and 25% of bound material is shown for all panels. (B) Immunoprecipitation of Sir2 coprecipitates Net1–HA3 and Fob1–Myc13 (lane 4). (Δ) sir2Δ cells; (+) SIR2+ cells. (C) Western blots showing that Fob1 and Net1 can physically associate in vivo in the absence of Sir2 (lanes 3,4,7,8). (D, left) A Coomassie-stained gel of purified GST–Control (lane 1) and GST–Fob1 (lane 2) proteins. The control protein (UAP56) is a human protein involved in mRNA splicing. (Right) Western blot indicating that subunits of RENT from whole-cell yeast extracts associate specifically with GST–Fob1 (lane 5) but not with the GST–Control protein (lane 4). Neither GST fusion protein interacts with actin (Act1) or transcriptional repressors that do not participate in rDNA silencing (Sir3, Sir4, and Tup1).