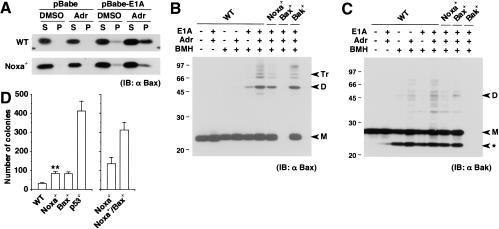
Figure 2.
Contribution of Noxa to activation of Bax and Bak. (A) Bax membrane insertion during E1A-dependent apoptosis. Control and E1A-expressing MEFs were treated with DMSO control buffer or adriamycin (0.25 μg/mL) for 6 h. A protein sample (10 μg) was separated into alkali-sensitive supernatant (S) and alkali-resistant pellet (P), and was loaded into the corresponding lane. Without stimulation, Bax molecules reside in the cytoplasm or are weakly attached to the surface of mitochondria, which are readily detached by alkali treatment. Thus, they are separated into the S fraction upon alkali extraction. When inserted into the mitochondrial membrane, Bax molecules acquire alkali resistance and are separated into the P fraction (Goping et al. 1998). The level of Bax molecules that acquired alkali resistance is lower in E1A-expressing Noxa-/- MEFs after adriamycin treatment than in similarly treated wild-type (WT) MEFs. IB indicates immunoblot. (B) Bax oligomerization during E1A-dependent apoptosis. Control and E1A-expressing MEFs were treated with DMSO control buffer or adriamycin (0.25 μg/mL) for 6 h, and subsequently, Bax oligomerization was analyzed through BMH cross-linking. A cross-linked protein sample (40 μg) was loaded into each lane. Markers M, D, and Tr correspond to the sizes of the monomer, dimer, and trimer, respectively. (C) Bakoligomerization during E1A-dependent apoptosis analyzed as in B. Markers M and D correspond to the sizes of the monomer and dimer, respectively. (ast;) An intramolecularly oligomerized Bak monomer. (D) Colony formation of MEFs expressing E1A and Ras on medium containing methylcellulose. Wild-type (WT) and Noxa-/- MEFs (left) as well as Noxa-/- and Noxa-/-/Bax-/- MEFs (right), were each prepared from the same litter. Values shown are means ± S.D. from triplicate samples. Results are representative of three independent experiments. (**) Noxa-/- MEFs generated a significantly increased number of colonies compared with wild-type MEFs (P < 0.01).