Figure 6.
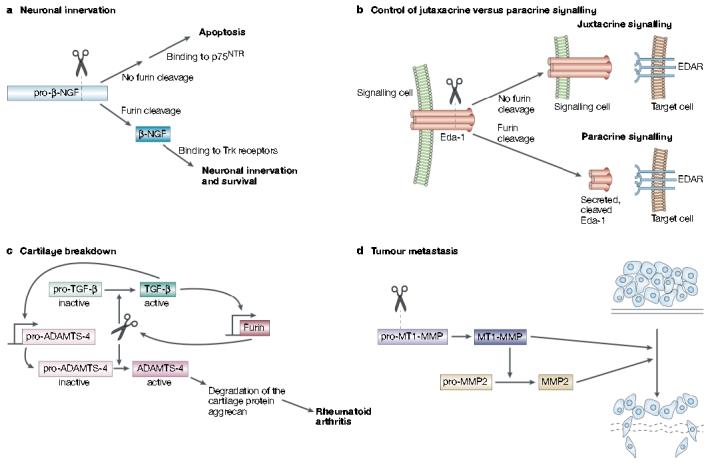
Furin in development, homeostasis and disease. a | Furin-mediated cleavage of pro-β-nerve growth factor (NGF) produces the 13-kDa β-NGF neurotrophin that binds to Trk receptors to promote synaptic innervation. By contrast, inhibition or sequestering of furin results in the secretion of pro-β-NGF that binds to the 75-kDa neurotrophin receptor (p75NTR) to promote cell-death pathways. b | Ectodysplasin-A (Eda-1) is a trimeric tumour necrosis factor family member that stimulates morphogenesis of ectodermal structures by activation of its receptor, EDAR, on target cells. Eda-1 can signal in a juxtacrine manner by binding to EDAR on adjacent cells. However, cleavage of membrane anchored Eda-1 by furin releases the ligand and enables it to signal through EDAR on distant cells in a paracrine manner. c | In synoviocytes, furin and transforming growth factor (TGF)-β participate in a positive feedback loop that results in elevated levels of ‘a disintegrin and metalloprotease with thrombospondin motifs-4’ (ADAMTS-4, or aggrecanase-1). Furin cleaves both pro-TGF-β and pro-ADAMTS-4 to yield the active growth factor and protease, respectively. The secreted mature form of TGF-β then binds to its receptor and, through a SMAD2 and mitogen-activated protein kinase (MAPK) convergent pathway, increases furin expression. The increased levels of furin lead to an increase in TGF-β, which creates a positive-feedback loop. In synoviocytes, TGF-β also stimulates the expression of pro-ADAMTS-4. So, because furin and TGF-β are in this positive loop, the levels of active ADAMTS-4 are greatly elevated, which leads to destruction of the cartilage protein aggrecan and hence to rheumatoid arthritis. d | Furin activates several membrane-type matrix metalloproteinases (MT-MMPs) that are involved in tumour formation and metastasis. Furin-activated MT-MMP1 activates MMP2 (gelatinase), which degrades the extracellular matrix, and MT-MMP1 also directly degrades extracellular matrix itself.
