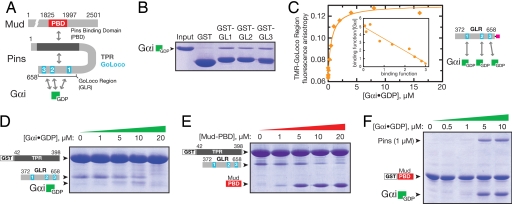
Fig. 1.
Pins intramolecular interaction regulates Gαi and Mud binding. (A) Domain structure of Pins and intra- and intermolecular interactions (PBD, region of Mud that binds Pins). (B) Each of the Pins GoLoco motifs can bind Gαi. Individual GST fusions of the three GoLocos bind Gαi·GDP at qualitatively similar levels. (C) The Pins GoLocos are intrinsically independent, equivalent Gαi binding sites. The extent of Gαi·GDP binding to the Pins GoLocos was monitored by the fluorescence anisotropy of a tetramethylrhodamine attached to a cysteine at its C terminus. The curve represents a model with three equivalent, independent binding sites of affinity Kd = 530 ± 80 nM. A Scatchard analysis is shown in Inset where the binding function is equal to the concentration of Pins-bound Gαi·GDP divided by the total concentration of Pins. (D) Gαi disrupts the Pins intramolecular interaction. In a qualitative “pull-down” assay, Gαi·GDP competes with the Pins GLR for binding to the Pins TPRs. Although Gαi ultimately disrupts the TPR–GLR interaction, at intermediate Gαi concentrations (5–10 μM), a Gαi–GLR–TPR complex can be formed, presumably those that result in occupation of GoLoco1 but not GoLoco2/3. At a higher concentration (20 μM), occupation of all three GoLoco motifs by Gαi interferes with the interaction of the GLR with the TPR region. Proteins are stained with Coomassie brilliant blue. (E) The Mud PBD disrupts the Pins intramolecular interaction. Binding of Mud to the Pins TPRs (as in B) competes with the Pins GoLocos. (F) Gαi increases the affinity of Pins for Mud. Full-length Pins binds weakly to the Mud PBD, but binding is enhanced by the presence of Gαi·GDP, indicating that Gαi and Mud bind cooperatively to Pins.