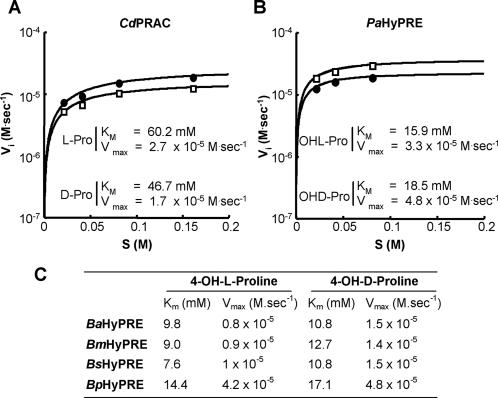
Figure 4. Kinetic parameters of Proline racemization and Hydroxyproline epimerization.
Progress of enzymatic activities was monitored polarimetrically, as described previously [8]. Initial rates were plotted in function of [S] and kinetic parameters determined with Kaleidagraph® program and Michaelis-Menten equation. Maximum rate (Vmax) and Michaelis-Menten constant (Km) were obtained at 37°C by incubation of 20 µg/ml of each recombinant protein with increasing concentrations of specific L- (closed circles) or D- (open squares) substrates. (A) PRAC activity is depicted for C. difficile; (B) HyPRE activity is depicted for P. aeruginosa. (C) Km and Vmax records of HyPRE reactions using L- or D- enantiomers were distinctively obtained with recombinant enzymes of B. abortus (BaHyPRE), B. melitensis (BmHyPRE), B. suis (BsHyPRE) and B. pseudomallei (BpHyPRE). TcPRACA : Km of 29 mM and Vmax of 5,3×10−5 M.sec−1 and TcPRACB : Km of 75 mM and Vmax of 2×10−4 M.sec−1.