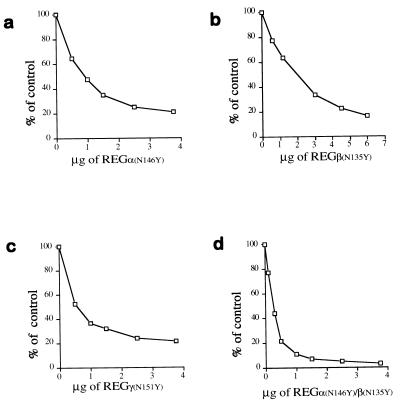
Figure 4.
Dominant negative properties of REG homologs with Asn-to-Tyr substitutions. (a) Inhibition of REGα-activated peptide hydrolysis by REGαN146Y. Proteasomes (170 ng) and 1.5 μg of REGα were mixed with increasing amounts of REGαN146Y, incubated at 37°C for 10 min, and then 50 μl of 200 μM Suc-LLVY-MCA was added to start the reactions. Product formation was measured as fluorescence units, and the results are expressed as a percentage of the activity in the absence of REGαN146Y. (b) Inhibition of REGβ’s activity by REGβN135Y. The assay was performed as described in a except that 3 μg of REGβ was added to each reaction. (c) Inhibition of REGγ’s activity by REGγN151Y. The assay was performed as described in a except that Suc-LRR-MCA was the substrate. (d) Inhibition of the activity of REG from human red blood cells by REGαN146Y/REGβN135Y. REGαN146Y/REGβN135Y heterooligomers were preformed by overnight incubation at 4°C and purified by gel filtration chromatography. Hrbc REG (1.5 μg) was added to each reaction, and increasing amounts of REGαN146Y/REGβN135Y were added prior to substrate. The enzymatic reaction was then performed as in a. Less inhibition was observed when recombinant wild-type REGα/β oligomers were challenged with REGαN146Y/REGβN135Y. Each point in all the figures represents the mean of three measurements. All inhibition experiments were performed independently at least twice by using REG proteins from different purifications.