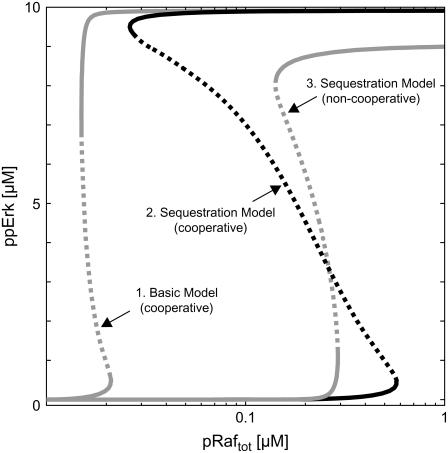
FIGURE 5.
Synergism of bistability mechanisms. A broad range of bistability is observed in the stimulus-response (curve 2) of the sequestration model (Fig. 1 D, black and gray arrows) if the second step of Mek-mediated Erk phosphorylation proceeds faster than the first (“positive cooperativity”). Such pronounced hysteresis can be explained by synergism of the feedback mechanism discussed in this article with that described by Markevich et al. (22), which arises from enzyme depletion effects in the Erk cycle. The gray lines correspond to the stimulus-response curves of reduced models, where one of the two feedback mechanisms was eliminated, and thereby directly demonstrate such synergism. Curve 1 depicts the stimulus response of the basic model (Fig. 1 D, black arrows), which is devoid of Mek sequestration into Raf-inaccessible complexes. Curve 3 corresponds to a sequestration model (Fig. 1 D, black and gray arrows), where positive cooperativity and enzyme-depletion effects in the Erk cycle are eliminated. See Supplementary Material for differential equations and kinetic parameters.