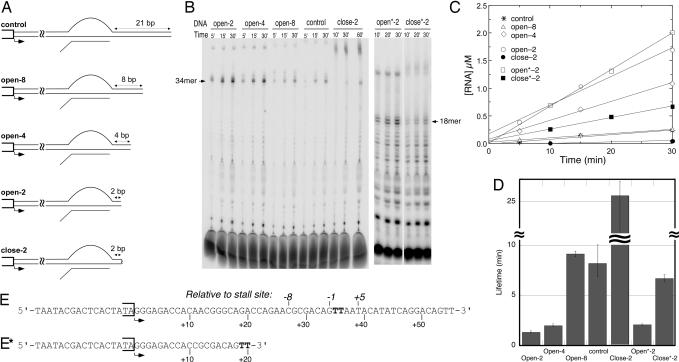
Fig. 2.
Contributions of downstream DNA to stability. (A) DNA constructs with varying lengths of downstream DNA. Sequences up to the halt site are identical for all constructs and allow halting at position +34 in the presence of GTP, ATP, and CTP. All constructs are fully duplex; the bubble in a halted complex is shown for clarity. In close-2 and close*-2, the end of the DNA is closed by an 18-atom polyethylene glycol spacer that covalently links the template and nontemplate strands, preventing full melting. (B) Denaturing gel electrophoresis of labeled RNA products at increasing time intervals. Reactions at 37°C contained 0.1 μM enzyme and 0.5 μM DNA (0.2/1.0 μM for the open*-2 and close*-2 constructs). (C) Stalled RNA products from B were quantified, and the data were plotted as a function of time. The slopes (velocities) divided by the concentration of enzyme were used as an estimate for the first-order rate constants (koff) for dissociation, the rate limiting step in turnover. (D) The rate constants were then used to calculate the half-lives of the halted elongation complexes by using t½ = ln(2)/koff. (E) The nontemplate strand sequence of the control construct (others have the same sequence truncated appropriately). (E*) The nontemplate strand sequence of the constructs open*-2 and close*-2.