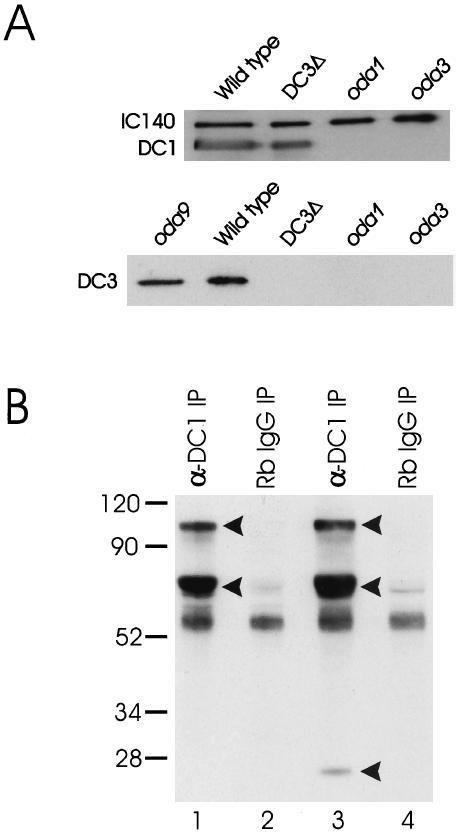
Figure 6.
(A) DC1 and DC2 assemble on the axoneme in the absence of DC3, but not vice versa. Top panel: Axonemes from wild type, the DC3-deletion strain (DC3Δ), oda1, and oda3 were isolated and analyzed by western blotting. oda1 is null for DC2; oda3 is null for DC1. The blot was probed with antibodies to DC1, DC2, and the inner arm IC, IC140 (used as a loading control). As expected, antibodies to DC1 detected protein in wild-type axonemes, but not in oda1 or oda3 axonemes, which lack the ODA-DC. Importantly, the antibody also detected protein in axonemes of the DC3-deletion strain. Essentially identical results were obtained with antibodies to DC2 (our unpublished results). These data indicate that DC1 and DC2 can assemble on the axoneme in the complete absence of DC3. Bottom panel: Axonemes from wild type, the DC3-deletion strain (DC3Δ), oda1, oda3, and oda9 were prepared as above (oda9 has a defect in IC1 and lacks outer dynein arms but retains the ODA-DC). The blot was probed with a polyclonal antibody to DC3. The antibody detects a single protein of Mr ∼25,000 in both oda9 and wild-type axonemes but detects no protein in DC3-null axonemes, indicating that it is specific for DC3. Axonemes from oda1 and oda3 do not contain DC3, indicating that DC3 assembly is dependent on the presence of DC1 and DC2. (B) Immunoprecipitation of the ODA-DC in the absence of Mg2+. The DC1 antibody was used to immunoprecipitate the ODA-DC from biotinylated 0.6 M KCl extracts of DC3-transformant (strain W215) and DC3-null axonemes. The immunoprecipitated proteins were then resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and detected using streptavidin-HRP. The anti-DC1 antibody immuno-precipitated three proteins of Mr ∼105,000, ∼70,000, and ∼25,000 (arrowheads) from DC3-transformant axonemal extracts (lane 3). These proteins, corresponding to DC1, DC2, and DC3, respectively, were not immunoprecipitated from DC3-transformant axonemal extracts using normal rabbit IgG (lane 4). In contrast, the anti-DC1 antibody immunoprecipitated only DC1 and DC2 (arrowheads) from the DC3-null axonemal extracts (lane 1). These proteins were not immunoprecipitated from DC3-null axonemal extracts using normal rabbit IgG (lane 2). These data confirm that a “partial” docking complex composed of DC1 and DC2 assembles on the axoneme when DC3 is missing and that transformation of the DC3-deletion strain with the DC3 gene restores DC3 to the ODA-DC. Numbers on left indicate molecular weight markers.