Figure 2. VCX-A Is an RNA-Binding Protein and Preferentially Binds the 5′ Cap.
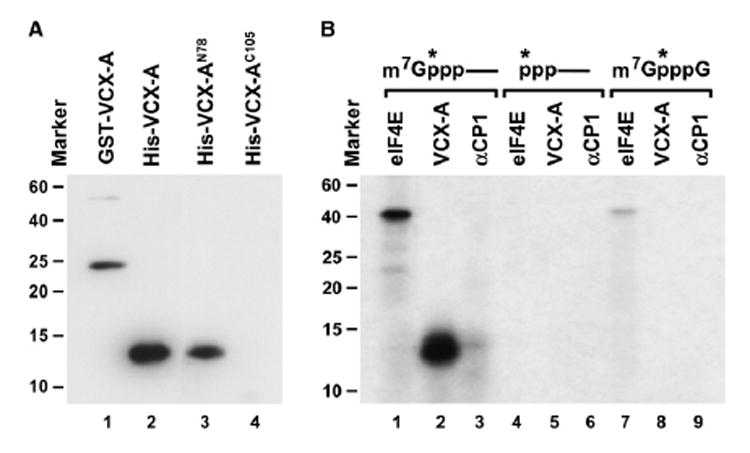
(A) Northwestern assay demonstrating the RNA-binding property of VCX-A. Ten picomoles of each recombinant protein consisting of the GST fusion or His-tagged full-length VCX-A protein (lane 1 and lane 2, respectively) and His-tagged N-terminal 78 amino acids of VCX-A (lane 3) or the C-terminal 105 amino acids of VCX-A (lane 4) was resolved by SDS-PAGE and transferred to nitrocellulose. 32P-uniform-labeled pcP RNA was used as an RNA substrate. Protein size markers are denoted on the left. The protein sizes correspond to the migration of each protein on SDS-PAGE gel stained by Coo-massie blue. The anomalous migration of the VCX-AN78 on SDS-PAGE gel is most likely a consequence of the variable charged nature of VCX-A. Where the N terminus is highly positively charged and the C terminus is highly negatively charged, the N terminus alone migrates slower than would be expected.
(B) VCX-A preferentially binds the cap of a capped RNA. UV crosslinking was carried out with three different substrates, all containing the 32P phosphate at the same position indicated by an asterisk. Twenty picomoles GST-eIF4E, 2 pmol His-VCX-A, and 20 pmol His-αCP1 recombinant proteins were incubated with cap-labeled pcP RNA (lanes 1–3), uncapped 5′ end-labeled pcP RNA (lanes 4–6), or 32P-labeled cap analog (lanes 7–9) at room temperature for 15 min. Reactions were subjected to UV irradiation for 10 min on ice with a 15 W germicidal lamp, subsequently treated with RNase cocktail, and resolved on SDS-PAGE gel.
