Figure 4.
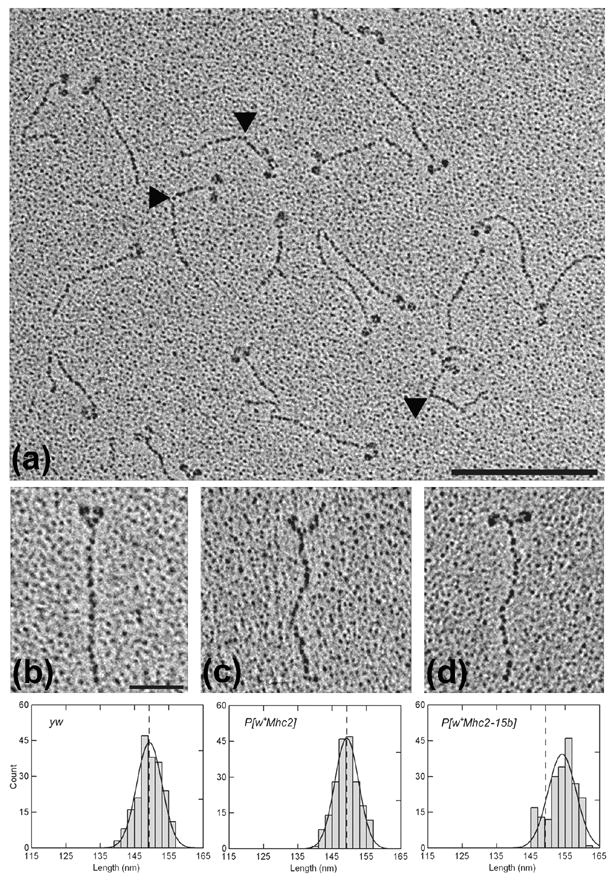
Electron micrographs of rotary shadowed myosin molecules from wild-type (yw), PwMhc2, and PwMhc2-15b hinge-switch Drosophila IFM. (a) A representative population of wild-type IFM myosin. Bar = 200 nm. Note the presence of molecules containing two clearly resolved N-terminal globular heads connected to a single C-terminal tail. A triangular, electron dense head to tail junction can be seen in several molecules. Furthermore, the extreme C-terminus of the rod can frequently be distinguished from the surrounding granular background. A sharp bend often found in the rod (denoted by black triangles) appears to represent the skip residue at the N-terminus of the S2/LMM hinge region.6 It is located 44 nm from the head to tail junction in both hinge A- and hinge B-containing molecules [44.02 ± 0.74 nm for yw (n = 54) and 43.98 ± 0.76 nm for 15b-47 (n = 55); mean ± SEM]. (b–d) Enlarged micrographs of single myosin molecules from yw (b), PwMhc2 (c), and PwMhc2-15b-47 (d) IFM that are suitable for rod contour length determinations. Bar = 50 nm. Over 200 myosin tails from each line were individually measured from the head to tail junction to the extreme C-terminus and averaged together to ascertain the mean contour rod lengths. As shown by the frequency distribution of rod lengths (lower panel), the mean contour lengths of yw and PwMhc2 tails (dotted lines) are the same and significantly shorter than the mean length of PwMhc2-15b tails.
