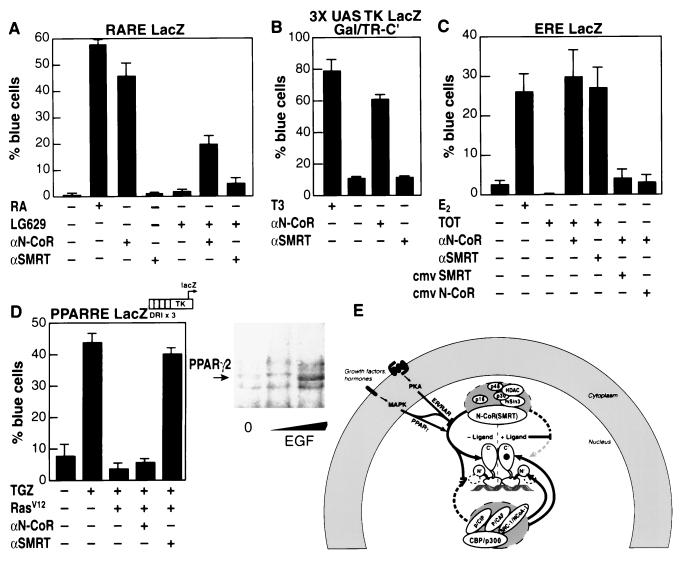
Figure 4.
N-CoR and SMRT complexes have receptor-specific roles. (A) A reporter under the control of retinoic acid receptor response elements was microinjected into Rat-1 cells, and effects of α-N-CoR or α-SMRT IgG were tested in the presence or absence of retinoic acid (RA) or the antagonist LG629. (B) A GAL fusion to the thyroid hormone receptor C terminus was microinjected, and effects of α-N-CoR or α-SMRT IgG were analyzed. (C) After microinjection of α-N-CoR, plasmid rescue experiments were performed as indicated. (D) CV-1 cells were injected with a plasmid encoding activated H-ras (val12), the indicated antisera, and a PPARγ (DR+1 site)-dependent TK promoter and treated with 1 μM troglitizone (TGZ) as indicated (Left). CV-1 cells were treated with increasing concentrations of EGF to stimulate MAP kinases (Right), and α-SMRT immunoprecipitations were performed from whole-cell extracts. Interactions were detected with antibodies against PPARγ2. (E) Model of regulation of nuclear receptor association with corepressor complexes. Both ligands and external signaling pathways regulate the association of specific corepressor and coactivator complexes with nuclear receptors. At least one member of a receptor homo- or heterodimer binds strongly, in the absence of ligand (or in the presence of antagonist for ER/PR), to the corepressor complex, localizing histone deacetylase activity to the promoter. This complex suppresses a constitutive N-terminal activation domain of the receptor. The corepressor complex is dismissed by agonist ligands, which allows recruitment of an acetylase-containing coactivator complex that interacts with both the receptor C-terminal AF-2 and the N-terminal AF-1 activation domains. Phosphorylation-dependent signaling pathways, initiated at the cell membrane, influence receptor activity by inhibiting the recruitment of the corepressor complex to steroid (ER/PR) and retinoid (RAR) receptors or, conversely, by stimulating its recruitment to peroxisome proliferator-activated receptor γ (PPARγ), increasing the recruitment of the coactivator complex.