Abstract
Alpha/beta-type small, acid-soluble proteins (SASP) of dormant spores of Bacillus subtilis bind to DNA and increase its resistance to a variety of damaging agents both in vivo and in vitro. When spores germinate, degradation of alpha/beta-type SASP is rapidly initiated by a sequence-specific protease, which is termed GPR. Three mutations have been introduced into the B. subtilis sspC gene, which codes for the wild-type alpha/beta-type SASP SspCwt; all three mutations change residues in the highly conserved sequence recognized by GPR. In one mutant protein (SspCV), residue 33 (Ser) was changed to Val; in the second (SspCDL), residues 30 and 31 (Glu and Ile) were changed to Asp and Leu, respectively; and in the third mutant protein (SspCDLV), residues 30, 31, and 33 were changed to Asp, Leu, and Val. All three mutant proteins were rapidly degraded by GPR during spore germination, and SspCDL and SspCDLV were degraded by GPR in vitro at rates 8 to 9% of that for SspCwt, although not exclusively at the single site cleaved by GPR in SspCwt. These results indicate (i) that the sequence specificity of GPR is broader than originally imagined and (ii) that GPR can cleave the sequence in SspCDLV. Since the latter sequence is identical to that cleaved during the proteolytic activation of GPR, this result further supports an autoprocessing model for GPR activation during sporulation. The properties of these mutant proteins were also examined, both in vivo in B. subtilis spores and in Escherichia coli and in vitro with purified protein. SspC(v) interacted with DNA similarly to SspC(wt) in vivo, resorting UV and heat resistance to spores lacking major alpha/beta-type SASP to the same extent as SspC(wt). In contrasst, SspC(DL) had much less effect on DNA properties in vivo and bound strongly only to poly(dG) . poly(dC) in vitro; SspC(DLV) exhibited only weak binding to poly(dG).poly(dC) in vitro. These results confirm the importance of the conserved primary sequence of alpha/beta-type SASP in the binding of these proteins to spore DNA and alteration of DNA properties and show further that the GRP recognition region in alpha/beta-type SASP plays some role in DNA binding.
Full text
PDF


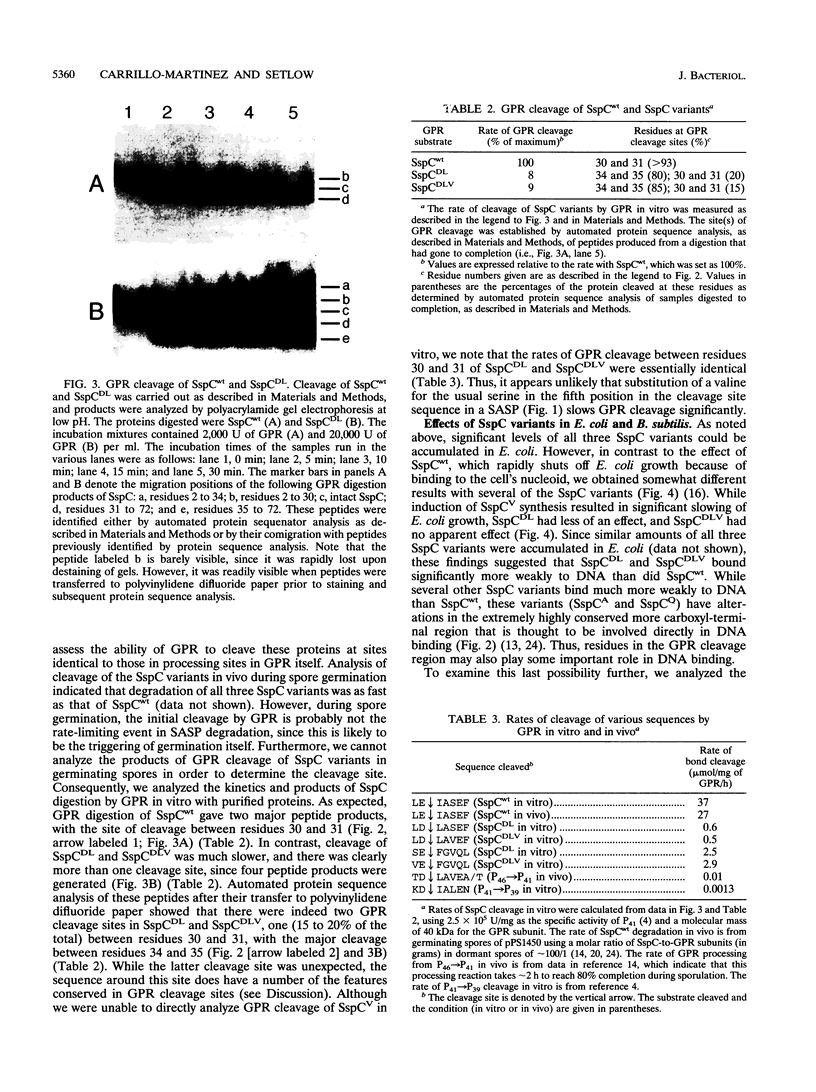
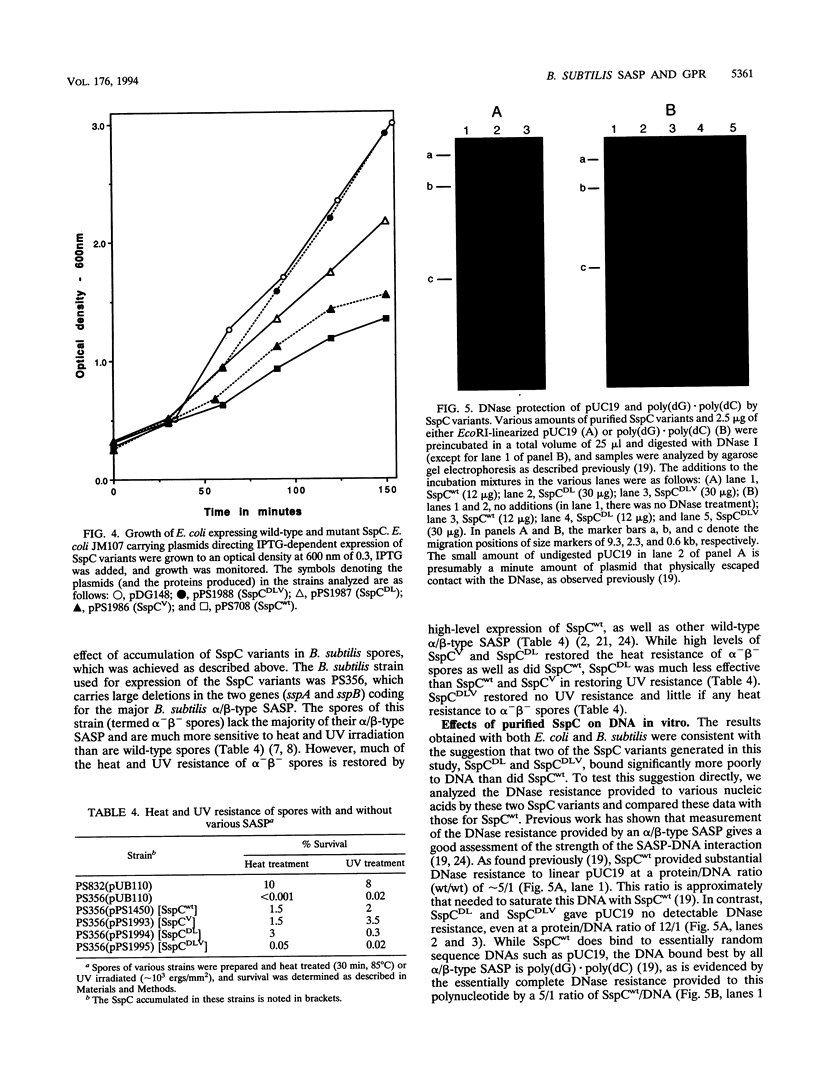


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Connors M. J., Setlow P. Cloning of a small, acid-soluble spore protein gene from Bacillus subtilis and determination of its complete nucleotide sequence. J Bacteriol. 1985 Jan;161(1):333–339. doi: 10.1128/jb.161.1.333-339.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhead H., Setlow B., Setlow P. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J Bacteriol. 1993 Mar;175(5):1367–1374. doi: 10.1128/jb.175.5.1367-1374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrick S., Setlow P. Expression of a Bacillus megaterium sporulation-specific gene during sporulation of Bacillus subtilis. J Bacteriol. 1983 Sep;155(3):1459–1462. doi: 10.1128/jb.155.3.1459-1462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illades-Aguiar B., Setlow P. Studies of the processing of the protease which initiates degradation of small, acid-soluble proteins during germination of spores of Bacillus species. J Bacteriol. 1994 May;176(10):2788–2795. doi: 10.1128/jb.176.10.2788-2795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illades-Aguiar B., Setlow P. The zymogen of the protease that degrades small, acid-soluble proteins of spores of Bacillus species can rapidly autoprocess to the active enzyme in vitro. J Bacteriol. 1994 Sep;176(17):5571–5573. doi: 10.1128/jb.176.17.5571-5573.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Setlow P. Different small, acid-soluble proteins of the alpha/beta type have interchangeable roles in the heat and UV radiation resistance of Bacillus subtilis spores. J Bacteriol. 1987 Aug;169(8):3633–3637. doi: 10.1128/jb.169.8.3633-3637.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Setlow P. Essential role of small, acid-soluble spore proteins in resistance of Bacillus subtilis spores to UV light. J Bacteriol. 1986 Jul;167(1):174–178. doi: 10.1128/jb.167.1.174-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Setlow B., Setlow P. Binding of DNA in vitro by a small, acid-soluble spore protein from Bacillus subtilis and the effect of this binding on DNA topology. J Bacteriol. 1990 Dec;172(12):6900–6906. doi: 10.1128/jb.172.12.6900-6906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Setlow B., Setlow P. Ultraviolet irradiation of DNA complexed with alpha/beta-type small, acid-soluble proteins from spores of Bacillus or Clostridium species makes spore photoproduct but not thymine dimers. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8288–8292. doi: 10.1073/pnas.88.19.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Setlow P. Dramatic increase in negative superhelicity of plasmid DNA in the forespore compartment of sporulating cells of Bacillus subtilis. J Bacteriol. 1990 Jan;172(1):7–14. doi: 10.1128/jb.172.1.7-14.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk P. G. A gene encoding a small, acid-soluble spore protein from alkaliphilic Bacillus firmus OF4. Gene. 1993 Mar 15;125(1):81–83. doi: 10.1016/0378-1119(93)90749-s. [DOI] [PubMed] [Google Scholar]
- Rao H., Mohr S. C., Fairhead H., Setlow P. Synthesis and characterization of a 29-amino acid residue DNA-binding peptide derived from alpha/beta-type small, acid-soluble spore proteins (SASP) of bacteria. FEBS Lett. 1992 Jun 29;305(2):115–120. doi: 10.1016/0014-5793(92)80876-i. [DOI] [PubMed] [Google Scholar]
- Sanchez-Salas J. L., Setlow P. Proteolytic processing of the protease which initiates degradation of small, acid-soluble proteins during germination of Bacillus subtilis spores. J Bacteriol. 1993 May;175(9):2568–2577. doi: 10.1128/jb.175.9.2568-2577.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Salas J. L., Sharon M., Setlow P. Effect of mutant small, acid-soluble spore proteins containing cysteine or tryptophan on DNA properties in vivo and in vitro. Biochimie. 1992 Jul-Aug;74(7-8):651–660. doi: 10.1016/0300-9084(92)90137-4. [DOI] [PubMed] [Google Scholar]
- Setlow B., Hand A. R., Setlow P. Synthesis of a Bacillus subtilis small, acid-soluble spore protein in Escherichia coli causes cell DNA to assume some characteristics of spore DNA. J Bacteriol. 1991 Mar;173(5):1642–1653. doi: 10.1128/jb.173.5.1642-1653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl Environ Microbiol. 1993 Oct;59(10):3418–3423. doi: 10.1128/aem.59.10.3418-3423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Sun D., Setlow P. Interaction between DNA and alpha/beta-type small, acid-soluble spore proteins: a new class of DNA-binding protein. J Bacteriol. 1992 Apr;174(7):2312–2322. doi: 10.1128/jb.174.7.2312-2322.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. I will survive: protecting and repairing spore DNA. J Bacteriol. 1992 May;174(9):2737–2741. doi: 10.1128/jb.174.9.2737-2741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- Stragier P., Bonamy C., Karmazyn-Campelli C. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell. 1988 Mar 11;52(5):697–704. doi: 10.1016/0092-8674(88)90407-2. [DOI] [PubMed] [Google Scholar]
- Tovar-Rojo F., Setlow P. Effects of mutant small, acid-soluble spore proteins from Bacillus subtilis on DNA in vivo and in vitro. J Bacteriol. 1991 Aug;173(15):4827–4835. doi: 10.1128/jb.173.15.4827-4835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]