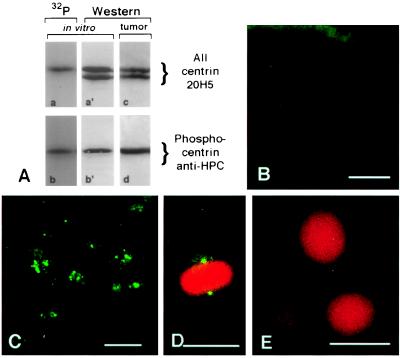
Figure 4.
Inappropriate phosphorylation of breast tumor centrosomes. (A) Specificity of αHPC antibodies for phosphocentrin. Western blot analysis of phosphorylated and nonphosphorylated bacterially expressed recombinant centrin (A, gel lanes a′ and b′) and autoradiography of the same gel lanes (A, gel lanes a and b). Comparison of the Western blot analysis (A, gel lane a′) by using mAb 20H5 that reacts with centrin regardless of phosphorylation status and the corresponding autoradiogram (A, gel lane a) demonstrates that the slower migrating centrin band is phosphorylated. Western blot analysis (A, gel lane b′) by using αHPC antibody to detect phosphorylated centrin demonstrates reactivity only with the slower migrating form of centrin and no reaction with the nonphosphorylated form. Analysis of whole cell extracts demonstrates that centrin is phosphorylated in breast tumor tissue (A, compare gel lanes c and d). (B) A section of a normal breast duct labeled with αHPC antibody demonstrates only a background level of staining in normal epithelial cells. (C) A section of a breast adenocarcinoma labeled with αHPC antibody demonstrates that centrosomes stain intensely, indicating that centrin is phosphorylated in tumor cells. (D–E) Immunofluorescence of the normal breast epithelial cell line MCF10A stained with αHPC antibody for phosphocentrin and propidium iodide for DNA. The spindle poles of a mitotic cell react strongly with αHPC antibody (D), whereas interphase cells show no labeling of their centrosomes (E). Tumor centrosome and mitotic MCF10A cell staining with αHPC are completely eliminated with a fivefold excess of competing phosphopeptide (data not shown). (Bar = 20 μm.)